NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Amifampridine is an orally available potassium channel blocker that increases acetylcholine in synaptic clefts of peripheral nerve endings and is used to treat the Lambert-Eaton myasthenic syndrome. Amifampridine is associated with a low rate of transient serum enzyme elevations during therapy but has not been linked with instances of clinically apparent acute liver injury.
Background
Amifampridine (am" i fam' pri deen) is a diaminopyridine that acts on peripheral potassium channels and is used to treat the Lambert-Eaton myasthenic syndrome, a rare form of myasthenia suspected to be autoimmune in nature. The inhibition of the potassium channel on neuromuscular junctions causes depolarization of the presynaptic membrane resulting in prolonged action potentials and increased release of acetylcholine in the synaptic cleft. This increase in acetylcholine alleviates some of the neuromuscular dysfunction of myasthenia which is caused by defects in acetylcholine signaling. In small open-label and randomized controlled trials, amifampridine was found to alleviate the myasthenic symptoms in patients with the rare Lambert-Eaton myasthenic syndrome, but did not reverse the condition or its associated autoimmunity. In 2018, amifampridine was approved as symptomatic therapy for Lambert-Eaton syndrome in adults and is available in scored tablets of 10 mg under the brand name Firdapse. In 2019, amifampridine was approved as symptomatic therapy for Lambert-Eaton syndrome in children and became available as scored 10 mg tablets under the brand name Fuzurgi. The recommended starting dose is 15 to 30 mg in 3 to 4 divided doses daily with dose increases of 5 mg and a maximum total dose of 80 mg daily. A reduced dose is recommended for children weighing less than 45 kilograms. Common side effects are paresthesia, upper respiratory symptoms, abdominal pain, nausea, diarrhea, headache, back pain, hypertension and muscle spasm. Rare, but potentially serious adverse events include seizures and hypersensitivity reactions.
Hepatotoxicity
Amifampridine has had limited clinical use, but adverse events have been largely neurologic and gastrointestinal. Serum ALT elevations were not reported in the prelicensure studies of amifampridine but were reported as occurring in a small proportion of patients in safety reviews by the Food and Drug Administration. Nevertheless, there have been no reports of clinically apparent liver injury associated with its use. Thus, liver injury from amifampridine must be rare if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury, but experience with its use is limited).
Mechanism of Injury
The mechanism by which amifampridine might cause liver injury is not known. It is extensively metabolized by the liver largely by N-acetyltransferase (NAT) to an inactive metabolite. The NAT gene is highly polymorphic and activity rates range from slow to fast. Persons who are slow acetylators have 3- to 5-fold higher plasma levels than those who are fast acetylators. Nevertheless, there is no clinical evidence that acetylation status affects the efficacy or safety of amifampridine to a major extent.
Drug Class: Myasthenia Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Amifampridine – Generic, Firdapse®, Ruzurgi®
DRUG CLASS
Myasthenia Agents
Product labeling at DailyMed, National Library of Medicine, NIH
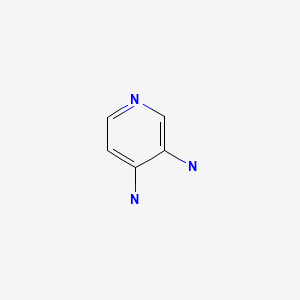
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Amifampridine | 54-96-6 | C5-H7-N3 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 15 May 2019
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Textbook of hepatotoxicity published in 1999, before the availability of amifampridine, does not discuss myasthenic syndromes and their therapy).
- Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013.(Textbook of hepatotoxicity published in 2013, does not discuss amifampridine or other drugs for myasthenic syndromes).
- Taylor P. Anticholinesterase Agents. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 163-75.(Textbook of pharmacology and therapeutics).
- FDA. https://www
.accessdata .fda.gov/scripts/cder/daf/ (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy; mentions that safety data were based upon 2 controlled withdrawal studies in 79 adults and that elevated liver enzymes occurred in 7 patients [13%], but all were self-limited and not accompanied by symptoms or jaundice). - Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to drugs used for myasthenic syndromes such as amifampridine).
- Verma S, Mazell SN, Shah DA. Amifampridine phosphate in congenital myasthenic syndrome. Muscle Nerve. 2016;54:809–10. [PubMed: 27348204](Two children [ages 4 and 5 years] with congenital myasthenic syndromes associated with mutations in the acetylcholine receptor had improvements in clinical symptoms [ptosis, dysarthria, energy level] with amifampridine treatment [10-20 mg daily], with only minor side effects; no mention of ALT levels or hepatotoxicity).
- Burns TM, Smith GA, Allen JA, Amato AA, Arnold WD, Barohn R, Benatar M, et al. Editorial by concerned physicians: Unintended effect of the orphan drug act on the potential cost of 3,4-diaminopyridine. Muscle Nerve. 2016;53:165–8. [PubMed: 26662952](Commentary by academic neurologists concerned about the approval of amifampridine [3,4-diaminopyridine] as an orphan drug for Lambert-Eaton myasthenic syndrome and the possible result of a very expensive therapy that would replace the reasonably priced compassionate use product).
- McEnany PJ. A response to a recent editorial by concerned physicians on 3,4-diaminopyridine. Muscle Nerve. 2017;55:138. [PubMed: 27756108](Response to the commentary by Burns [2016] argues that the benefit of an FDA approved therapy for Lambert-Eaton syndrome far outweighs the possible negative effect of increase price).
- Schoser B, Eymard B, Datt J, Mantegazza R. Lambert-Eaton myasthenic syndrome (LEMS): a rare autoimmune presynaptic disorder often associated with cancer. J Neurol. 2017;264:1854–63. [PubMed: 28608304](Review of the history of discovery, epidemiology, pathophysiology, clinical features, electromyographic findings, autoimmune serology, diagnosis, management and therapy of Lambert-Eaton myasthenic syndrome; mentions that amifampridine may be effective for its symptomatic treatment [increasing neurotransmission] but that immunosuppressive therapy is needed to decrease disease progression).
- Sanders DB, Juel VC, Harati Y, Smith AG, Peltier AC, Marburger T, Lou JS, et al. Dapper Study Team. 3,4-diaminopyridine base effectively treats the weakness of Lambert-Eaton myasthenia. Muscle Nerve. 2018;57:561–8. [PMC free article: PMC5900968] [PubMed: 29280483](Among 32 adults with Lambert-Eaton myasthenia on compassionate use 3,4-diaminopyridine who were either continued on treatment or switched to placebo for at least 3 months, objective measurements of myasthenic symptoms worsened in those who were withdrawn and not in those who continued on therapy, and “there were no clinically meaningful laboratory value changes”).
- Bonanno S, Pasanisi MB, Frangiamore R, Maggi L, Antozzi C, Andreetta F, Campanella A, et al. Amifampridine phosphate in the treatment of muscle-specific kinase myasthenia gravis: a phase IIb, randomized, double-blind, placebo-controlled, double crossover study. SAGE Open Med. 2018;6:2050312118819013. [PMC free article: PMC6299310] [PubMed: 30574306](Among 10 patients with muscle-specific kinase myasthenia gravis who were treated with amifampridine or placebo in a double-cross over study, myasthenia symptoms improved on amifampridine and deteriorated on placebo, and the only drug-specific adverse event was transient paresthesia; there were “no relevant differences between the two groups for…blood chemistries…over the duration of the study”).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Amifampridine tablets for the treatment of Lambert-Eaton myasthenic syndrome.[Expert Rev Clin Pharmacol. 2019]Review Amifampridine tablets for the treatment of Lambert-Eaton myasthenic syndrome.Mantegazza R. Expert Rev Clin Pharmacol. 2019 Nov; 12(11):1013-1018. Epub 2019 Oct 22.
- Amifampridine to treat Lambert-Eaton myasthenic syndrome.[Drugs Today (Barc). 2020]Amifampridine to treat Lambert-Eaton myasthenic syndrome.Oh SJ. Drugs Today (Barc). 2020 Oct; 56(10):623-641.
- Review Amifampridine for the Management of Lambert-Eaton Myasthenic Syndrome: A New Take on an Old Drug.[Ann Pharmacother. 2020]Review Amifampridine for the Management of Lambert-Eaton Myasthenic Syndrome: A New Take on an Old Drug.Yoon CH, Owusu-Guha J, Smith A, Buschur P. Ann Pharmacother. 2020 Jan; 54(1):56-63. Epub 2019 Jul 18.
- Review Treatment for Lambert-Eaton myasthenic syndrome.[Cochrane Database Syst Rev. 2003]Review Treatment for Lambert-Eaton myasthenic syndrome.Maddison P, Newsom-Davis J. Cochrane Database Syst Rev. 2003; (2):CD003279.
- Review Amifampridine for the treatment of Lambert-Eaton myasthenic syndrome.[Expert Rev Clin Immunol. 2019]Review Amifampridine for the treatment of Lambert-Eaton myasthenic syndrome.Oh SJ. Expert Rev Clin Immunol. 2019 Oct; 15(10):991-1007. Epub 2019 Sep 30.
- Amifampridine - LiverToxAmifampridine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...