NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Apalutamide is a third generation, oral nonsteroidal antiandrogen used to treat nonmetastatic castration-resistant prostate cancer. Apalutamide is associated with a low rate of serum enzyme elevation during therapy but has not been linked to cases of clinically apparent liver injury with jaundice.
Background
Apalutamide (a pa lut' a mide) is a small molecule androgen receptor antagonist which binds to the intracellular receptor and prevents its translocation to the nucleus and subsequent DNA binding, thereby blocking its activity. Therapy with apalutamide lowers residual testosterone levels after surgical castration in men with prostate cancer and has been shown to prolong metastasis free survival in men with castration-resistant prostate cancer with rising levels of prostate-associated antigen (PSA) without measurable metastatic disease. Apalutamide was approved for use in the United States in 2018 and current indications include metastatic, castration-sensitive prostate cancer and non-metastatic castration-resistant prostate cancer. Apalutamide is available as tablets of 60 and 240 mg under brand name Erleada. The recommended initial dose is 240 mg daily with subsequent dose reduction for intolerance. It should be administered with a gonadotropin-releasing hormone (GnRH) analog or after bilateral orchiectomy to insure optimal androgen suppression. Common side effects include symptoms of androgen deficiency including fatigue, diarrhea, nausea, anorexia, weight loss, constipation, joint and muscle pain, hot flushes, headaches, dizziness, and edema. Rare, but potentially serious side effects associated with long term therapy include seizures, osteoporosis, falls, bone fractures, severe cutaneous adverse events, embryo-fetal toxicity, and cardiovascular events.
Hepatotoxicity
In prelicensure controlled trials of apalutamide, serum aminotransferase elevations were uncommon and generally transient and mild, not requiring dose modification. Clinically apparent liver injury with jaundice attributable to apalutamide was not reported in the preregistration trials and is not mentioned as an adverse event in the product label. Since the approval and general clinical use of apalutamide, there have been no publications or descriptions of the clinical features of hepatotoxicity with jaundice associated with its use. The first and second generation androgen receptor blockers, flutamide, nilutamide, and bicalutamide, have all been linked to instances of hepatitis-like liver injury with jaundice that can be severe and even fatal. However, such cases have not been described with apalutamide and other third generation androgen receptor antagonists. Thus, clinically apparent liver injury due to apalutamide must be rare, if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The possible cause of liver injury due to apalutamide therapy is unknown. While first and second generation antiandrogens have been implicated in causing liver injury, the more potent third general factors have not. Apalutamide is extensively metabolized in the liver predominantly by CYP 2C8 and 3A and is an inducer of CYP 3A4. While coadministration of apalutamide with substrates of CYP 3A4 and with modulators of CYP 2C8 and 3A4 may result in drug-drug interactions, the effects are relatively modest.
Outcome and Management
The liver injury linked to apalutamide therapy has been generally mild, consisting of transient and asymptomatic elevations in serum aminotransferase levels and rarely requiring dose modification or discontinuation. Apalutamide has not been linked to cases of acute liver failure, chronic hepatitis or vanishing bile duct syndrome. There is no information on cross sensitivity to hepatic injury between apalutamide and other antiandrogens, such as flutamide, bicalutamide, or abiraterone.
Drug Class: Antineoplastic Agents, Antiandrogens
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Apalutamide – Erleada®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
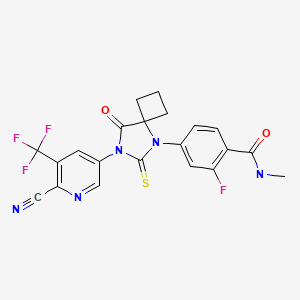
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Apalutamide | 956104-40-8 | C21-H15-F4-N5-O2-S |
|
ANNOTATED BIBLIOGRAPHY
References updated: 15 March 2023
Abbreviations: LHRH, luteinizing hormone releasing hormone, PSA, prostate-specific antigen.
- Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999 before the availability of apalutamide).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam, Elsevier, 2013, p. 541-68.(Review of hepatotoxicity of cancer chemotherapeutic agents; apalutamide is not discussed).
- Isaacs C, Wellstein A, Riegel AT. Hormones and related agents in the therapy of cancer. Natural products in cancer chemotherapy: hormones and related agents. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1237-48.(Textbook of pharmacology and therapeutics discusses the androgen receptor antagonists flutamide, bicalutamide, nilutamide and enzalutamide, but not apalutamide).
- FDA. https://www
.accessdata .fda.gov/scripts/cder/daf/ (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy; mentions that hepatic laboratory abnormalities were uncommon and the single instance of clinically apparent liver injury with jaundice appeared to be due to progressive hepatic metastases rather than apalutamide toxicity). - Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. [PMC free article: PMC2981508] [PubMed: 19359544](Description of development of unique antiandrogen molecules that block the translocation of the androgen receptor to the nucleus and the transcriptional activity of the receptor).
- Smith MR, Antonarakis ES, Ryan CJ, Berry WR, Shore ND, Liu G, Alumkal JJ, et al. Phase 2 study of the safety and antitumor activity of apalutamide (ARN-509), a potent androgen receptor antagonist, in the high-risk nonmetastatic castration-resistant prostate cancer cohort. Eur Urol. 2016;70:963–70. [PMC free article: PMC5568792] [PubMed: 27160947](Among 51 patients with castration-resistant prostate cancer and rising levels of PSA who were treated with apalutamide [240 mg daily], 89% had a 50% decline in PSA levels within 12 weeks and common side effects were fatigue [61%], diarrhea [43%], nausea [39%], joint and back pain [22%], hot flush [20%] and abdominal pain [18%], and no serious adverse events occurred; no mention of ALT elevations or hepatotoxicity).
- Rathkopf DE, Antonarakis ES, Shore ND, Tutrone RF, Alumkal JJ, Ryan CJ, Saleh M, et al. Safety and antitumor activity of apalutamide (ARN-509) in metastatic castration-resistant prostate cancer with and without prior abiraterone acetate and prednisone. Clin Cancer Res. 2017;23:3544–51. [PMC free article: PMC5543693] [PubMed: 28213364](Among 46 men with castration-resistant prostate cancer and rising PSA levels treated with apalutamide [240 mg daily], 88% had at least a 50% decline in PSA levels by 12 weeks, side effects were mostly mild, and there were no treatment-related serious adverse events; no mention of ALT elevations or hepatotoxicity).
- Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, Olmos D, et al. SPARTAN Investigators. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408–18. [PubMed: 29420164](Among 1207 men with castration-resistant prostate cancer with rising levels of PSA but without detectable metastases in a randomized, placebo controlled trial, median metastasis-free survival was longer with apalutamide than placebo [40.5 vs 16.2 months], and adverse events that were more frequent with apalutamide included fatigue [30% vs 21%], rash [24% vs 6%], weight loss [16% vs 6%], falls [16% vs 9%], fractures [12% vs 7%], hypothyroidism [8% vs 2%] and seizures [0.2% vs none]; no mention of ALT elevations or hepatotoxicity).
- Beaver JA, Kluetz PG, Pazdur R. Metastasis-free survival – a new end point in prostate cancer trials. N Engl J Med. 2018;378:2458–60. [PubMed: 29949489](Editorial from FDA on the implications of the approval of apalutamide and application of metastasis-free survival [rather than overall survival] as an end-point in assessing efficacy of new agents for prostate cancer, stressing the importance of balancing benefit with safety and risk of adverse events).
- Al-Salama ZT. Apalutamide: first global approval. Drugs. 2018;78:699–705. [PubMed: 29626324](Review of the structure, mechanism of action, history of development, clinical efficacy and safety of apalutamide; mentions that overall and serious adverse event rates were similar in apalutamide- and placebo-treated subjects; no mention of ALT elevations or hepatotoxicity).
- Apalutamide (Erleada) for prostate cancer. Med Lett Drugs Ther. 2018;60(1551):e124–e125. [PubMed: 30036351](Concise review of the efficacy, safety and costs of apalutamide shortly after its approval in the US mentions side effects reported by Smith et al. [2018], but does not mention hepatotoxicity or ALT elevations).
- Crawford ED, Schellhammer PF, McLeod DG, Moul JW, Higano CS, Shore N, Denis L, et al. Androgen receptor targeted treatments of prostate cancer: 35 years of progress with antiandrogens. J Urol. 2018;200:956–966. [PubMed: 29730201](Review of the development of antiandrogen therapies of prostate cancer starting with discovery of the androgen sensitive nature of prostate cancer, the effects of orchiectomy, followed by the development of androgen receptor antagonists, first generation agents flutamide and nilutamide, second generation agent bicalutamide and third generation agents enzalutamide, apalutamide and darolutamide that have more potent androgen receptor inhibition).
- Halabi S, Jiang S, Terasawa E, Garcia-Horton V, Ayyagari R, Waldeck AR, Shore N. Indirect comparison of darolutamide versus apalutamide and enzalutamide for nonmetastatic castration-resistant prostate cancer. J Urol. 2021;206:298–307. [PubMed: 33818140](Comparison of outcome and adverse events in published randomized, placebo-controlled trials of darolutamide, apalutamide and enzalutamide for castration-resistant prostate cancer suggested that efficacy as assessed by improvement in metastasis-free survival was similar for all three agents, but that darolutamide therapy was associated with lower rates of adverse events, particularly fatigue, rash, falls, seizures, fractures and cognitive disorders; no mention of ALT elevations or hepatotoxicity).
- Saad F, Efstathiou E, Attard G, Flaig TW, Franke F, Goodman OB Jr, Oudard S, et al. ACIS Investigators. Apalutamide plus abiraterone acetate and prednisone versus placebo plus abiraterone and prednisone in metastatic, castration-resistant prostate cancer (ACIS): a randomised, placebo-controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 2021;22:1541–1559. [PMC free article: PMC9377412] [PubMed: 34600602](Among 982 men with metastatic prostate cancer treated with abiraterone [1000 mg with prednisone] with or without apalutamide [240 mg], radiographic progression free survival was longer with combination therapy [22.6 vs 16.6 months], but overall survival was not different [67% vs 69%] and adverse events were more frequent with the combination, including hypertension [17% vs 10%] and ALT elevations [12% vs 4%], but there were no hepatic deaths or mention of clinically apparent liver injury).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Darolutamide.[LiverTox: Clinical and Researc...]Review Darolutamide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Enzalutamide.[LiverTox: Clinical and Researc...]Review Enzalutamide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Apalutamide for the treatment of patients with castration-resistant prostate cancer.[Drugs Today (Barc). 2018]Review Apalutamide for the treatment of patients with castration-resistant prostate cancer.Hauke R. Drugs Today (Barc). 2018 Oct; 54(10):585-590.
- [Apalutamide, Erleada®].[Rev Med Liege. 2022][Apalutamide, Erleada®].Sautois B, Denis C. Rev Med Liege. 2022 Oct; 77(10):609-615.
- Health-related Quality of Life at the SPARTAN Final Analysis of Apalutamide for Nonmetastatic Castration-resistant Prostate Cancer Patients Receiving Androgen Deprivation Therapy.[Eur Urol Focus. 2022]Health-related Quality of Life at the SPARTAN Final Analysis of Apalutamide for Nonmetastatic Castration-resistant Prostate Cancer Patients Receiving Androgen Deprivation Therapy.Oudard S, Hadaschik B, Saad F, Cella D, Basch E, Graff JN, Uemura H, Dibaj S, Li S, Brookman-May SD, et al. Eur Urol Focus. 2022 Jul; 8(4):958-967. Epub 2021 Sep 1.
- Apalutamide - LiverToxApalutamide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...