NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Bismuth is a brittle, silvery-white metal that is used in paints, cosmetics, electronics, and a few over-the-counter pharmaceuticals, usually as bismuth subsalicylate or subcitrate, primarily to treat diarrhea and digestive disorders. Currently, the major medical use of bismuth is as a component of quadruple therapy of Helicobacter pylori infection. There is scant evidence that bismuth, even in high doses, causes serum aminotransferase elevations or clinically apparent liver injury.
Background
Bismuth is silvery white metal similar to tin and lead that has many commercial uses and has long been used to treat indigestion and diarrhea. Bismuth is currently widely used in combination with antibiotics and omeprazole as “quadruple” therapy for eradication of Helicobacter pylori (H. pylori) infection, a major cause of peptic ulcer disease and possibly gastric carcinoma. Bismuth is found naturally as sulfide and oxide forms and is a common byproduct of lead refining. Bismuth is not an essential element and is rarely present in foods and then only in trace amounts. Nevertheless, bismuth has been used as a pharmaceutical agent typically to treat diarrhea and previously for various infections including syphilis. Currently, the major use of bismuth is in combination with at least two antibiotics and omeprazole in “quadruple” therapy for eradication of Helicobacter pylori. The mechanism of action of bismuth is not well defined, but in addition to providing a coating to ulcers and inflamed issue, bismuth has direct activity against H. pylori. Approved forms of bismuth include bismuth subcitrate potassium [140 mg] in fixed combination with metronidazole [125 mg] and tetracycline [125 mg] generically and under the brand name Pylera, and as bismuth subsalicylate [262 mg] in fixed combination with metronidazole [250 mg] and tetracycline [500 mg] generically and under the brand name Helidac. These fixed combinations were approved in doses of 2 tablets or capsules 4 times daily in combination with omeprazole (20 mg twice daily) or an H2 blocker for 10 or 14 days. Bismuth subsalicylate is also available without prescription, generically in tablets of 262 mg to combine with separate prescriptions for antibiotics and omeprazole to treat H. pylori or use as monotherapy for diarrhea and indigestion, given as 2 tablets every 30 to 60 minutes as needed but not to exceed 16 tablets in any 24 hours or to continue for more than 2 days. A popular nonprescription form of bismuth subsalicylate is “Pepto-Bismol” which is given in doses of 525 mg (30 mL) every 30 or 60 minutes until symptomatic resolution not to exceed 8 doses in one day or more than two days of therapy. Bismuth subsalicylate is rapidly disassociated in the stomach allowing salicylate to be absorbed, while bismuth is minimally absorbed. Bismuth is generally well tolerated, its major side effects being darkening of the stool and greyish discoloration of the tongue and teeth.
Bismuth toxicity has been described but is now rare. It typically presents with neurologic symptoms including change in personality, abnormal behavior, muscle twitching, myoclonal jerks, ataxia, confusion, somnolence, and stupor. Such patients have typically been taking oral tablets or solution of bismuth intermittently for months if not years, but with high doses and when given parenterally, the onset can be within days of starting bismuth. The diagnosis can be suspected based upon the neurologic symptoms and the physical finding of discoloration of the tongue and teeth. Plasma bismuth levels are high (typically above 50 μg/L with normal levels being less than 5 μg/L). After stopping bismuth, plasma levels decrease gradually accompanied by resolution of symptoms. Most patients return to baseline levels of function but in severe cases, there can be residual neurologic deficits. Excessive bismuth subsalicylate can also cause salicylism with tinnitus and weakness, but bismuth therapy is usually recommended for short periods only.
Hepatotoxicity
In large, controlled trials of bismuth-based quadruple therapy in patients with H. pylori infection, adverse event rates were similar in patients receiving therapy that included bismuth as those on triple H. pylori regimens without bismuth. Monotherapy with bismuth for digestive complaints and diarrhea has not been linked to significant adverse events or elevations in serum ALT or bilirubin, but few studies have assessed liver tests routinely during the brief use of bismuth for episodes of diarrhea or to prevent travelers diarrhea. Further, in the many case reports of bismuth neurotoxicity, there was no mention of hepatotoxicity, and where this information was provided, liver tests were reported to be normal.
Despite the lack of evidence of liver injury in recent studies of bismuth therapy, several reports of both liver and kidney injury have been reported in the past which were attributed to bismuth. Most of these reports dealt with therapy of syphilis using injections of arsenicals and bismuth. Many of the cases were exposed to other possible hepatotoxins (arsenic), but all were reported from the 1920s and 1940s and may have been acute hepatitis B or C contracted because of inadequate sterilization of needles and syringes used in the injection therapy.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Drug Class: Trace Elements and Metals
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Bismuth – Generic, Helidac®, Pylera®
DRUG CLASS
Trace Elements and Metals
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
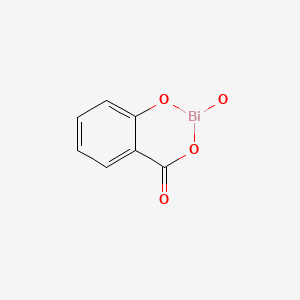
| Bismuth Subsalicylate | 14882-18-9 | C7-H6-Bi-O |
|
ANNOTATED BIBLIOGRAPHY
References updated: 03 April 2024
Abbreviations used: H. pylori, Helicobacter pylori.
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999; bismuth is not discussed).
- Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013.(Textbook on hepatotoxicity; bismuth is not discussed).
- Byrns MC, Penning TM. Treatment of metal exposure. Environmental toxicology: carcinogens and heavy metals. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1311-5.(Textbook of pharmacology and therapeutics).
- Stokes JH, Ruedemann R, Lemon WS. Epidemic infectious jaundice and its relation to the therapy of syphilis. Arch Intern Med 1920; 26: 521-543. Not in PubMed.(Between 1916 and 1920, there were 70 cases of jaundice arising during arsphenamine therapy of syphilis at the Mayo Clinic, but the frequency increased markedly during that time [1000-fold] suggesting an epidemic of hepatitis rather than hepatitis from therapy, an idea which was also supported by a lack of uniform latency to onset after starting therapy and lack of recurrence when therapy was resumed as well as the similarity of symptoms [particularly prodromal symptoms of fever, arthritis, and rash], laboratory tests, and timing between jaundice in epidemics occurring concurrently in Minnesota).
- Nomland R, Skolnik EA, McLellan LL. Jaundice from bismuth compounds used in the therapy of syphilis. JAMA 1938; 11; 19-21. Not in PubMed.(Among 75 cases of jaundice occurring during therapy of syphilis, 32 were attributed to bismuth subsalicylate, after 11 to more than 30 injections, beginning within 6 weeks of the last dose, ten having received bismuth only, the remainder also receiving arsphenamine. The authors state that the injury is indistinguishable from “catarrhal jaundice” [acute viral hepatitis] and in several cases bismuth was restarted without recurrence of jaundice, factors that suggest that the injury was due to acute hepatitis B or C transmitted by inadequately sterilized reusable syringes or needles).
- Wolllmann J. Acute necrosis of the liver following sodium bismuth thioglycolate administration. Am J Syph Gonorrhea Vener Dis 1940; 29: 330-336. Not in PubMed.(Two case reports of newborns developing acute hepatic necrosis and fulminant liver failure after injections of bismuth for treatment of syphilis, one shortly after the first injection and the other after 10 injections over almost a year of therapy).
- Kulchar HV, Reynolds WJ. Bismuth hepatitis. JAMA 1942; 120: 343-346. Not in PubMed.(Among 1032 inmates at San Quentin receiving treatment for syphilis between 1936 and 1942, 144 developed jaundice during bismuth therapy [typically after 4-8 of the 60 injections required]; all were male, 103 white, ages 19 to 58 years, 117 also received arsenicals).
- Burns R, Thomas DW, Barron VJ. Reversible encephalopathy possibly associated with bismuth subgallate ingestion. Br Med J. 1974;1(5901):220-3. [PMC free article: PMC1633100] [PubMed: 4818163](Description of 5 patients who developed neurologic symptoms of tremulousness, confusion, ataxia, and myoclonic jerks, 6 months to 6 years after colorectal cancer surgery and long term intermittent treatment with oral bismuth subgallate; one patient died and one redeveloped symptoms upon restarting bismuth).
- Lowe DJ. Adverse effects of bismuth subgallate. A further report from the Australian Drug Evaluation Committee. Med J Aust 1974; 2: 664-6. Not in PubMed.4453225 [PubMed: 4453225](Summary of 29 patients who developed bismuth encephalopathy between 1966 and 1972 in Australia after treatment with oral bismuth subgallate daily for a variable period [weeks to years] as a means of controlling the consistency and odor of stool in patients with ileostomy and colostomy, most resolving within 1-3 months of stopping bismuth, but some with persistent neurologic symptoms; no mention of serum aminotransferase elevations or hepatotoxicity).
- Henderson IWD. Warning against products containing bismuth subsalicylate. Can Med Assoc J. 1980;123:848. [PMC free article: PMC1704921] [PubMed: 7437988](Expression of caution in using bismuth subsalicylate because of detectable levels of salicylate, which might be problematic in patients receiving anticoagulants or other salicylates).
- Hasking GJ, Duggan JM. Encephalopathy from bismuth subsalicylate. Med J Aust. 1982;2:167. [PubMed: 7132858](60 year old man with intermittent diarrhea developed inappropriate behavior, confusion, muscle twitching, and incontinence after using bismuth subsalicylate for several years with a blood bismuth level of 72 μg/L [normal less than 5 μg/L] and who recovered completely within a month of stopping bismuth).
- Bianchi Porro G, Lazzaroni M, Cortvriendt WR. Maintenance therapy with colloidal bismuth subcitrate in duodenal ulcer disease. Digestion. 1987;37 Suppl 2:47-52. [PubMed: 3305118](Among 39 adults with healed duodenal ulcer treated with daily bismuth subcitrate, there were mild side effects only and no patient developed bismuth encephalopathy or significant elevations in bismuth levels).
- Bradley B, Singleton M, Lin Wan Po A. Bismuth toxicity--a reassessment. J Clin Pharm Ther. 1989;14:423-41. [PubMed: 2693474](Extensive review of the toxicity of bismuth salts, focusing upon subsalicylate and subcitrate, including melanosis, oral discoloration, skeletal problems, nephrotoxicity, hepatotoxicity, and neurotoxicity, mentions 180 published cases of liver toxicity, 140 of which were also exposed to arsphenamine and commenting that other hepatotoxins could account for all the cases reported).
- Dunk AA, Prabhu U, Tobin A, O'Morain C, Mowat NA. The safety and efficacy of tripotassium dicitrato bismuthate (De-Nol) maintenance therapy in patients with duodenal ulceration. Aliment Pharmacol Ther. 1990;4:157-62. [PubMed: 2104081](Among 79 adults with healed duodenal ulcers treated with maintenance bismuth subcitrate [120 mg daily] for up to 12 months, therapy was well tolerated, there were no discontinuations for adverse events, and routine biochemistry tests showed no evidence of hepatotoxicity).
- Jungreis AC, Schaumburg HH. Encephalopathy from abuse of bismuth subsalicylate (Pepto-Bismol). Neurology. 1993;43:1265. [PubMed: 8192812](67 year old woman developed tremor, confusion, and intermittent delirium, 18 months after starting use of large doses of an oral solution of bismuth subsalicylate [Pepto-Bismol] for episodes of abdominal pain, plasma bismuth was 36 μg/L six weeks after stopping bismuth, and the neurologic symptoms resolving 6 months later).
- Youngman L, Harris S. BIPP madness; an iatrogenic cause of acute confusion. Age Ageing. 2004;33:406-7. [PubMed: 15082418](81 year old man developed acute confusion two days after nasal packing and surgery for epistaxis which was found to be attributable to bismuth toxicity, the source being bismuth iodoform paraffin paste [BIPP] used for nasal packing; serum “biochemistry” tests were reported to be normal).
- Ford AC, Malfertheiner P, Giguere M, Santana J, Khan M, Moayyedi P. Adverse events with bismuth salts for Helicobacter pylori eradication: systematic review and meta-analysis. World J Gastroenterol. 2008;14:7361-70. [PMC free article: PMC2778120] [PubMed: 19109870](Systematic review of 35 controlled trials of bismuth containing regimens [for 7 to 56 days] for eradication of Helicobacter pylori in 4763 patients found no difference in overall adverse event rates, individual adverse event rates, or rates of discontinuations for adverse events; no mention of liver injury or hepatotoxicity).
- Malfertheiner P, Bazzoli F, Delchier JC, Celiñski K, Giguère M, Rivière M, Mégraud F; Pylera Study Group. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet. 2011;377(9769):905-13. Erratum in: Lancet. 2011 Nov 19;378(9805):1778. [PubMed: 21345487](Controlled trial of quadruple [with bismuth] vs triple therapy [omeprazole and two antibiotics] for eradication of H. pylori infection in 440 adults demonstrated superiority of the quadruple regimen [80% vs 55%], with no difference in total and serious adverse event rates and no discontinuations for liver related events; bismuth levels increased but all were well below toxic levels and fell after stopping).
- Delchier JC, Malfertheiner P, Thieroff-Ekerdt R. Use of a combination formulation of bismuth, metronidazole and tetracycline with omeprazole as a rescue therapy for eradication of Helicobacter pylori. Aliment Pharmacol Ther. 2014;40:171-7. [PubMed: 24863854](Among 49 adults with H. pylori infection who had failed a course of triple therapy and were treated with quadruple therapy using bismuth, metronidazole, tetracycline, and omeprazole for 10 days, the eradication rate was 95% and adverse event rate 67%, but only one patient discontinued therapy early and “no clinically significant changes in laboratory tests were noted”).
- Borbinha C, Serrazina F, Salavisa M, Viana-Baptista M. Bismuth encephalopathy- a rare complication of long-standing use of bismuth subsalicylate. BMC Neurol. 2019;19:212. [PMC free article: PMC6714398] [PubMed: 31464594](44 year old woman developed abnormal behavior, confusion, postural instability, and myoclonic jerks having used bismuth subsalicylate [Pepto-Bismol] irregularly for 20 years for gastrointestinal complaints [serum bismuth 260 μg/L, liver tests normal], slowing improving as serum levels of bismuth fell and was back to baseline function 3 months later; finding of greyish discoloration of teeth gradually resolving).
- Silva C, Merim S, Sevivas R, Mota J, Leitão A. Bismuth subcitrate, metronidazole and tetracycline - a rare cause of drug-induced liver injury. Eur J Case Rep Intern Med. 2023;10:004119. [PMC free article: PMC10705836] [PubMed: 38077712](37 year old man with H. pylori infection developed nausea, abdominal pain, and fever 5 days after starting triple therapy with bismuth subcitrate, metronidazole and tetracycline [bilirubin 3.0 mg/dL, ALT 839 U/L, Alk P 136 U/L, INR 1.4], which resolved within 4 weeks of stopping therapy, the cause being unclear but perhaps due to metronidazole).
- Moss SF, Shah SC, Tan MC, El-Serag HB. Evolving concepts in Helicobacter pylori management. Gastroenterology. 2024;166:267-283. [PMC free article: PMC10843279] [PubMed: 37806461](Review of current recommendations for management of H. pylori infection states: “retrospective studies of H. pylori eradication, although sparse, suggest that bismuth-quadruple therapy for 10-14 days is likely the current best choice for empiric therapy in the U.S.”; no mention of adverse events, ALT elevations, or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- A Triple and Quadruple Therapy with Doxycycline and Bismuth for First-Line Treatment of Helicobacter pylori Infection: A Pilot Study.[Helicobacter. 2015]A Triple and Quadruple Therapy with Doxycycline and Bismuth for First-Line Treatment of Helicobacter pylori Infection: A Pilot Study.Ciccaglione AF, Cellini L, Grossi L, Manzoli L, Marzio L. Helicobacter. 2015 Oct; 20(5):390-6. Epub 2015 Mar 20.
- A Randomized Controlled Trial Shows that both 14-Day Hybrid and Bismuth Quadruple Therapies Cure Most Patients with Helicobacter pylori Infection in Populations with Moderate Antibiotic Resistance.[Antimicrob Agents Chemother. 2...]A Randomized Controlled Trial Shows that both 14-Day Hybrid and Bismuth Quadruple Therapies Cure Most Patients with Helicobacter pylori Infection in Populations with Moderate Antibiotic Resistance.Tsay FW, Wu DC, Yu HC, Kao SS, Lin KH, Cheng JS, Wang HM, Chen WC, Sun WC, Tsai KW, et al. Antimicrob Agents Chemother. 2017 Nov; 61(11). Epub 2017 Oct 24.
- Amoxicillin or tetracycline in bismuth-containing quadruple therapy as first-line treatment for Helicobacter pylori infection.[Gut Microbes. 2020]Amoxicillin or tetracycline in bismuth-containing quadruple therapy as first-line treatment for Helicobacter pylori infection.Bang CS, Lim H, Jeong HM, Shin WG, Choi JH, Soh JS, Kang HS, Yang YJ, Hong JT, Shin SP, et al. Gut Microbes. 2020 Sep 2; 11(5):1314-1323. Epub 2020 May 2.
- Review Guidelines for the treatment of Helicobacter pylori in the pediatric population.[Ann Pharmacother. 1997]Review Guidelines for the treatment of Helicobacter pylori in the pediatric population.Robinson DM, Abdel-Rahman SM, Nahata MC. Ann Pharmacother. 1997 Oct; 31(10):1247-9.
- Review Bismuth therapy in gastrointestinal diseases.[Gastroenterology. 1990]Review Bismuth therapy in gastrointestinal diseases.Gorbach SL. Gastroenterology. 1990 Sep; 99(3):863-75.
- Bismuth - LiverToxBismuth - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...