NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Budesonide is a corticosteroid that undergoes high first pass elimination by the liver so that systemic levels after oral administration are minimal. Budesonide has been used orally for several immune mediated gastrointestinal and liver diseases and as nasal spray or by inhalation for allergic rhinitis, asthma and chronic obstructive lung disease. Neither inhalant nor oral budesonide has been linked to serum enzyme elevations during therapy or to convincing instances of clinically apparent acute liver injury.
Background
Budesonide (bue des' oh nide) is a synthetic corticosteroid that has a high first pass elimination by the liver (~90%) so that after oral administration systemic exposure is minimal. Budesonide has been used to replace conventional corticosteroids given by inhalant or orally. Budesonide nasal spray and inhalants have been used to treat allergic rhinitis, asthma and bronchospasm occurring in patients with chronic obstructive pulmonary disease. Budesonide tablets have been used to replace conventional corticosteroids as the therapy of gastrointestinal immune mediated diseases such as ulcerative colitis, Crohn disease, collagenous colitis and autoimmune hepatitis. In many of these situations, oral budesonide was found to be equivalent to oral prednisone in efficacy, but better tolerated because of a reduction in glucocorticoid side effects. Budesonide was approved for use initially in 1996 as a nasal spray and solution for inhalation. In 2001, oral budesonide was approved as therapy for inflammatory bowel disease. Currently, budesonide is available for oral administration as capsules of 3 mg generically and under the brand names Entocort EC and Uceris (among others). The recommended dose is 9 mg daily. Current indications are limited to mild-to-moderate forms of ulcerative colitis and Crohn disease. Oral budesonide has also been evaluated as therapy of autoimmune hepatitis and collagenous colitis with reports of benefit, but has not been officially approved for these uses. Budesonide is also available as nasal sprays and solution for inhalation in many formulations generically and under brand names such as Pulmicort, Rhinocort and Symbicort. Approved indications for the inhalation forms of budesonide include allergic rhinitis, asthma and chronic obstructive pulmonary disease with an asthmatic component. Side effects of budesonide are not common and most are reported to be similar in rate with placebo or comparator therapies. The typical corticosteroid adverse events of weight gain, moon facies, buffalo hump, worsening of glaucoma, cataracts, osteopenia and others are not common with budesonide therapy and the majority of patients treated have no or only mild evidence of adrenal suppression.
Hepatotoxicity
Long term therapy with budesonide has not been linked to elevations in serum enzyme levels, and in clinical trials rates of ALT elevations were similar with budesonide as with placebo treatment. In controlled trials, there were no reported cases of clinically apparent liver injury associated with its use. Unlike conventional systemically administered corticosteroids, budesonide has not been linked to episodes of reactivation of hepatitis B. Budesonide has been used in severe autoimmune liver diseases without evidence that it causes worsening of liver injury. Because it can improve serum aminotransferase elevations in patients with autoimmune hepatitis, its withdrawal may be followed by rebound elevations as also occurs with conventional corticosteroid therapy. In addition, there has been a single case report of acute serum aminotransferase elevations during budesonide therapy that resolved when the drug was stopped, but documentation was limited and the patient was on multiple other potentially hepatotoxic drugs.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which budesonide might cause serum aminotransferase elevations or liver injury is not known. Glucocorticoids can cause liver injury, but generally only when given in high systemic doses, unlikely to be achieved by oral budesonide.
Drug Class: Hormones, Corticosteroids
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Budesonide – Generic, Entocort®, Uceris®
DRUG CLASS
Corticosteroids
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Budesonide | 51333-22-3 | C25-H34-O6 |
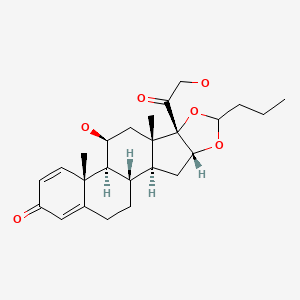
|
| Prednisolone | 50-24-8 | C21-H28-O5 |
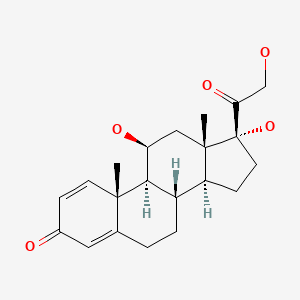
|
ANNOTATED BIBLIOGRAPHY
References updated: 30 October 2020
Abbreviations: IBD, inflammatory bowel disease; COPD, chronic obstructive pulmonary disease; LABA, long-acting beta2 agonists; LAMA, long-acting muscarinic antagonists.
- Zimmerman HJ. Corticosteroids. Drugs to treat rheumatic/musculospastic disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 541.(Textbook of hepatotoxicity published in 1999; discusses conventional corticosteroids, but does not mention budesonide).
- Chitturi S, Farrell GC. Corticosteroids. Adverse effects of hormones and hormone antagonists on the liver. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 613-4.(Review of liver injury from corticosteroids; mentions hepatic steatosis and acute liver injury following high doses of corticosteroids [usually methylprednisolone], but does not discuss budesonide).
- Schimmer BP, Funder JW. Adrenocortical steroids: ACTH, adrenal steroids, and pharmacology of the adrenal cortex. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1215-333.(Textbook of pharmacology and therapeutics).
- Intranasal budesonide for allergic rhinitis. Med Lett Drugs Ther. 1994;36(926):63–4. [PubMed: 8015513](Concise review of the mechanism of action, clinical efficacy, safety and costs of budesonide shortly after its approval in the US as an intranasal formulation to treat allergic rhinitis; no mention of hepatotoxicity or ALT elevations).
- Greenberg GR, Feagan BG, Martin F, Sutherland LR, Thomson AB, Williams CN, Nilsson LG, et al. Oral budesonide as maintenance treatment for Crohn's disease: a placebo-controlled, dose-ranging study. Canadian Inflammatory Bowel Disease Study Group. Gastroenterology. 1996;110:45–51. [PubMed: 8536887](Among 105 patients with Crohn disease treated with budesonide [3 or 6 mg daily] or placebo for 1 year, there were delays in onset of relapse in patients receiving budesonide, but rates of relapse at 1 year were the same in all groups and glucocorticoid side effects were higher with budesonide).
- Campieri M, Ferguson A, Doe W, Persson T, Nilsson LG. Oral budesonide is as effective as oral prednisolone in active Crohn's disease. The Global Budesonide Study Group. Gut. 1997;41:209–14. [PMC free article: PMC1891473] [PubMed: 9301500](Among 178 patients with Crohn disease treated with budesonide [9 mg daily or 4.5 mg twice daily] or prednisolone [40 mg daily] for 12 weeks, clinical remissions occurred in 60% of patients receiving prednisolone or budesonide once daily, but in only 42% on budesonide twice daily; while side effects except for moon face were similar in all groups, no mention of ALT elevations or hepatotoxicity).
- Ferguson A, Campieri M, Doe W, Persson T, Nygård G. Oral budesonide as maintenance therapy in Crohn's disease--results of a 12-month study. Global Budesonide Study Group. Aliment Pharmacol Ther. 1998;12:175–83. [PubMed: 9692692](Among 75 patients with Crohn disease in clinical remission treated with oral budesonide [3 or 6 mg] or placebo daily for 12 months, relapse rates were similar [48% and 46% vs 60%] while rates of glucocorticoid side effects were somewhat higher [23% and 46% vs 15%]; no mention of ALT elevations or hepatotoxicity).
- Leuschner M, Maier KP, Schlichting J, Strahl S, Herrmann G, Dahm HH, Ackermann H, et al. Oral budesonide and ursodeoxycholic acid for treatment of primary biliary cirrhosis: results of a prospective double-blind trial. Gastroenterology. 1999;117:918–25. [PubMed: 10500075](Among 20 patients with primary biliary cirrhosis treated with ursodiol and either budesonide or placebo for up to two years, improvements in serum enzymes and liver histology were greater in those receiving budesonide, but bone density deteriorated more).
- Czaja AJ, Lindor KD. Failure of budesonide in a pilot study of treatment-dependent autoimmune hepatitis. Gastroenterology. 2000;119:1312–6. [PubMed: 11054389](Among 10 patients with autoimmune hepatitis dependent upon prednisone therapy to maintain remission, only 3 could be maintained on budesonide, the other seven requiring conversion back to prednisone; exacerbations of liver disease were attributed to glucocorticoid withdrawal rather than hepatotoxicity of budesonide).
- Angulo P, Batts KP, Jorgensen RA, LaRusso NA, Lindor KD. Oral budesonide in the treatment of primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:2333–7. [PubMed: 11007238](Among 21 patients with sclerosing cholangitis treated with oral budesonide [9 mg daily] for 12 months, there were slight but nonsignificant decreases in Alk P and AST levels, but no changes in liver histology and appearance of glucocorticoid side effects in several patients and marked loss of bone mass as assessed by DEXA scanning).
- van Hoogstraten HJ, Vleggaar FP, Boland GJ, van Steenbergen W, Griffioen P, Hop WC, van Hattum J, et al. Budesonide or prednisone in combination with ursodeoxycholic acid in primary sclerosing cholangitis: a randomized double-blind pilot study. Belgian-Dutch PSC Study Group. Am J Gastroenterol. 2000;95:2015–22. [PubMed: 10950051](Among 18 patients with sclerosing cholangitis on ursodiol who were treated with budesonide [3 or 9 mg] or prednisone [10 mg] daily for 8 weeks, neither symptoms nor liver tests improved with budesonide therapy, while side effects were considered transient and minor except in one patient who developed autoimmune hepatitis requiring prednisone therapy during withdrawal of budesonide treatment).
- Angulo P, Jorgensen RA, Keach JC, Dickson ER, Smith C, Lindor KD. Oral budesonide in the treatment of patients with primary biliary cirrhosis with a suboptimal response to ursodeoxycholic acid. Hepatology. 2000;31:318–23. [PubMed: 10655252](Among 22 patients with primary biliary cirrhosis and an incomplete response to ursodiol who were treated with budesonide [9 mg daily] for 1 year, there were marginal improvements in Alk P, but continued progression of disease and worsening bone density and 2 patients developed glucocorticoid side effects; no worsening of serum enzyme levels during therapy).
- Nebulized budesonide for asthma in children. Med Lett Drugs Ther. 2001;43(1096):6–7. [PubMed: 11177221](Concise review of the clinical efficacy, adverse events and costs of nebulized budesonide shortly after its approval for use in children with asthma in the US; no mention of ALT elevations or hepatotoxicity).
- Budesonide (Entocort EC) for Crohn's disease. Med Lett Drugs Ther. 2002;44(1122):6–8. [PubMed: 11856950](Concise review of the mechanism of action, clinical efficacy, adverse events and costs of oral budesonide shortly after its approval for use in Crohn disease in the US; mentions that glucocorticoid side effects are less with budesonide than prednisone, but still occur as does suppressive effects on adrenal function; no mention of hepatotoxicity).
- Sagir A, Wettstein M, Oette M, Erhardt A, Häussinger D. Budesonide-induced acute hepatitis in an HIV-positive patient with ritonavir as a co-medication. AIDS. 2002;16:1191–2. [PubMed: 12004282](A 48 year old man with chronic HIV infection and rectal cancer developed marked ALT elevations [peak 1010 U/L; bilirubin and Alk P not given] 19 days after starting 5-ASA and budesonide for radiation colitis, which resolved after stopping budesonide and did not recur with restarting anti-HIV medications and 5-ASA; liver biopsy showed no necrosis or inflammation).
- Escher JC., European Collaborative Research Group on Budesonide in Paediatric IBD. Budesonide versus prednisolone for the treatment of active Crohn's disease in children: a randomized, double-blind, controlled, multicentre trial. Eur J Gastroenterol Hepatol. 2004;16:47–54. [PubMed: 15095852](Among 48 children with Crohn disease treated with budesonide or prednisolone, clinical remissions at week 8 occurred in similar rates [55% vs 71%] in both groups, but corticosteroid side effects [moon faces, acne] and adrenal suppression were less with budesonide).
- Sandborn WJ, Löfberg R, Feagan BG, Hanauer SB, Campieri M, Greenberg GR. Budesonide for maintenance of remission in patients with Crohn's disease in medically induced remission: a predetermined pooled analysis of four randomized, double-blind, placebo-controlled trials. Am J Gastroenterol. 2005;100:1780–7. [PubMed: 16086715](Combined analysis of 380 patients with Crohn disease participating in 4 clinical trials of maintenance of remission with budesonide [3 or 6 mg daily] vs placebo found median time to relapse was longer with 6 mg of budesonide [268 days] than 3 mg [170 days] or placebo [154 days], while adverse events included easy bruising in 5-10%, acne in 5-10% and moon face in 3-4%).
- Rautiainen H, Kärkkäinen P, Karvonen AL, Nurmi H, Pikkarainen P, Nuutinen H, Färkkilä M. Budesonide combined with UDCA to improve liver histology in primary biliary cirrhosis: a three-year randomized trial. Hepatology. 2005;41:747–52. [PubMed: 15754377](Among 77 patients with primary biliary cirrhosis treated with budesonide and ursodiol or ursodiol alone for 3 years, serum enzymes improved in both groups, while liver fibrosis improved slightly with the combination but worsened with ursodiol alone; 8 patients reported glucocorticoid side effects which led to early discontinuation in one).
- Miehlke S, Madisch A, Bethke B, Morgner A, Kuhlisch E, Henker C, Vogel G, et al. Oral budesonide for maintenance treatment of collagenous colitis: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2008;135:1510–6. [PubMed: 18926826](Among 77 patients with primary biliary cirrhosis treated with ursodiol and budesonide vs ursodiol alone for 3 years, liver histology improved more frequently with the combination while serum Alk P and ALT levels improved in both groups).
- Budesonide/formoterol (Symbicort) for asthma. Med Lett Drugs Ther. 2008;50(1279):9–11. [PubMed: 18264031](Concise summary of the efficacy, safety, drug interactions and costs of the inhalant combination of budesonide and formoterol shortly after its approval in the US for asthma; side effects were mostly local but some glucocorticoid side effects occurred; no mention of ALT elevations or hepatotoxicity).
- Bonderup OK, Hansen JB, Teglbjaerg PS, Christensen LA, Fallingborg JF. Long-term budesonide treatment of collagenous colitis: a randomised, double-blind, placebo-controlled trial. Gut. 2009;58:68–72. [PubMed: 18669576](Among 46 patients who achieved a clinical remission in collagenous colitis on oral budesonide [9 mg daily for 6 weeks], the relapse rate was 65% among 23 who stopped therapy compared to only 26% who remained on 6 mg daily of budesonide; there were no serious adverse events and no mention of ALT elevations or hepatotoxicity).
- Manns MP, Woynarowski M, Kreisel W, Lurie Y, Rust C, Zuckerman E, Bahr MJ, et al. European AIH-BUC-Study Group. Budesonide induces remission more effectively than prednisone in a controlled trial of patients with autoimmune hepatitis. Gastroenterology. 2010;139:1198–206. [PubMed: 20600032](Among 207 patients with newly diagnosed autoimmune hepatitis treated with budesonide [6 or 9 mg daily] or prednisone [40 mg initially], complete responses were more frequent with budesonide than prednisone [47% vs 18%], and glucocorticoid side effects were less [52% vs 72%]; no mention of hepatotoxicity).
- Safety of inhaled corticosteroids in chronic obstructive pulmonary disease (COPD). Med Lett Drugs Ther. 2010;52(1339):41–2. [PubMed: 20508579](Concise review of the safety and efficacy of two combinations of corticosteroids and beta-2 agonists in chronic obstructive pulmonary disease; mentions that inhaled corticosteroids may be associated with an increased risk of pneumonia and oral candidiasis; no mention of ALT elevations or hepatotoxicity).
- Budesonide (Uceris) for ulcerative colitis. Med Lett Drugs Ther. 2013;55(1412):23–4. [PubMed: 23507873](Concise review of the efficacy, adverse effects, drug interactions and costs of oral budesonide shortly after its approval for use in ulcerative colitis in the US; does not mention liver related side effects).
- Woynarowski M, Nemeth A, Baruch Y, Koletzko S, Melter M, Rodeck B, Strassburg CP, et al. European Autoimmune Hepatitis-Budesonide Study Group. Budesonide versus prednisone with azathioprine for the treatment of autoimmune hepatitis in children and adolescents. J Pediatr. 2013;163:1347–53.e1. [PubMed: 23810723](Among 46 children with newly diagnosed autoimmune hepatitis treated with budesonide [6 or 9 mg daily] or prednisolone [40 mg daily initially], complete response rates were similar in the two groups [16% vs 15%], although the decrease in serum ALT levels was faster with prednisolone; no mention of exacerbations or flares of hepatitis with treatment).
- Mieli-Vergani G, Vergani D. Budesonide for juvenile autoimmune hepatitis? Not yet. J Pediatr. 2013;163:1246–8. [PubMed: 23932214](Commentary on Woynarowsk et al. [2013] questioning the role of budesonide in pediatric autoimmune hepatitis, which tends to be more severe than in adults and in whom rapid control of the disease is desirable).
- Iborra M, Alvarez-Sotomayor D, Nos P. Long-term safety and efficacy of budesonide in the treatment of ulcerative colitis. Clin Exp Gastroenterol. 2014;7:39–46. [PMC free article: PMC3921089] [PubMed: 24523594](Review of the efficacy and safety of oral budesonide in ulcerative colitis; does not mention serum enzyme elevations or hepatotoxicity).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to budesonide or methylprednisone or other corticosteroids).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 2 were attributed to high dose methylprednisolone, but none to budesonide or other corticosteroids).
- Rubin DT, Sandborn WJ, Bosworth B, Zakko S, Gordon GL, Sale ME, Rolleri RL, et al. Budesonide foam has a favorable safety profile for inducing remission in mild-to-moderate ulcerative proctitis or proctosigmoiditis. Dig Dis Sci. 2015;60:3408–17. [PMC free article: PMC4621699] [PubMed: 26386854](Summary of 5 clinical trials of budesonide rectal foam for ulcerative proctitis found that side effects were similar between budesonide and placebo therapy and there was no evidence of pituitary-adrenal suppression, indicating a low systemic exposure to corticosteroids).
- Sherlock ME, MacDonald JK, Griffiths AM, Steinhart AH, Seow CH. Oral budesonide for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2015;(10):CD007698. [PMC free article: PMC9239584] [PubMed: 26497719](Systematic review of the literature concluded that budesonide with or without mesalamine is superior to placebo in inducing remission in ulcerative colitis, and that it is less likely to cause adrenal suppression and side effects than adequate amounts of conventional corticosteroids).
- Lichtenstein GR, Travis S, Danese S, D'Haens G, Moro L, Jones R, Huang M, et al. Budesonide MMX for the induction of remission of mild to moderate ulcerative colitis: a pooled safety analysis. J Crohns Colitis. 2015;9:738–46. [PMC free article: PMC4736820] [PubMed: 26094251](Among 835 patients with ulcerative colitis enrolled in 3 controlled trials of budesonide [9, 6 or 3 mg] or placebo daily for 8 weeks, serious adverse events were similar in all groups [2.0-2.7%] as were glucocorticoid related events).
- D'Haens G. Systematic review: second-generation vs. conventional corticosteroids for induction of remission in ulcerative colitis. Aliment Pharmacol Ther. 2016;44:1018–29. [PubMed: 27650488](In a systematic review of 21 studies of conventional corticosteroids vs those that target the site of inflammation of ulcerative colitis [small intestine and colon], budesonide and beclomethasone were judged to be superior to placebo and similar to conventional corticosteroids, but with “a more favorable safety and tolerability profile”).
- Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, Dransfield MT, et al. IMPACT Investigators. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671–80. [PubMed: 29668352](Among 10355 patients with COPD treated with inhaled glucocorticoids [CS: fluticasone], a long acting muscarinic antagonist [LAMA: umeclidinium] and a long-acting beta-2 agonist [LABA: vilanterol], triple therapy was associated with lower rates of moderate and severe exacerbations than dual therapy, although episodes of pneumonia were more frequent; no mention of ALT elevations or hepatotoxicity in extended analyses of adverse events).
- Trelegy Ellipta--a three-drug inhaler for COPD. Med Lett Drugs Ther. 2018;60(1547):86–8. [PubMed: 29913467](Concise summary of mechanism of action, clinical efficacy, safety and costs of a three-drug inhaler approved for use in COPD that includes a CS, LAMA and LABA, mentions that inclusion of corticosteroids yielded higher rates of clinical response but higher rates of pneumonia; no mention of ALT elevations or hepatotoxicity).
- OTC drugs for seasonal allergies. Med Lett Drugs Ther. 2019;61(1570):57–60. [PubMed: 31169808](Concise summary of the mechanism of action, clinical efficacy, safety and costs of over-the-counter drugs used to treat seasonal allergies such as allergic rhinitis, includes discussion of intranasal corticosteroids, considered the most effective agents for seasonal allergies, side effects of which can include irritation, dryness, burning and bleeding of nasal mucosa as well as sore throat and headache; no mention of ALT elevations or hepatotoxicity).
- Prado NMBL, Messias GC, Santos GO Junior, Nunes VS, Schinonni MI, Paraná R. Prospective monitoring of drug use: drug-induced liver injury in a primary healthcare center. Arq Gastroenterol. 2019;56:390–3. [PubMed: 31721973](Among 149 patients followed in a primary care center in Brazil, three were suspected to have developed liver injury due to a medication including nimesulide, budesonide and valacyclovir marked by moderate elevations in ALT levels [176-182 U/L], normal Alk P [46-52 U/L], normal bilirubin [0.3-0.4 mg/dL] and no symptoms, resolving rapidly on drug discontinuation).
- Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, Trivedi R, et al. ETHOS Investigators. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383:35–48. [PubMed: 32579807](Among 8529 patients with COPD treated with inhalants with or without budesonide, moderate or severe exacerbations of COPD were less frequent with budesonide inhalants while episodes of pneumonia were more frequent [3.5% to 4.5% vs 2.3%], and there were no significant changes in clinical laboratory test results).
- Bari K, Shah SA, Kaiser TE, Cohen RM, Anwar N, Kleesattel D, Sherman KE. Safety and efficacy of budesonide for liver transplant immune suppression: results of a pilot phase 2a trial. Liver Transpl. 2020;26:1430–40. [PMC free article: PMC7606621] [PubMed: 32602616](Among 20 patients undergoing liver transplantation treated postoperatively with budesonide in tapering doses for 12 weeks compared to 20 matched controls treated with tapering doses of prednisone, rates of acute cellular rejection were similar [5% vs 5%] while budesonide was associated with lower rates of new-onset diabetes [0% vs 15%] and opportunistic infections [0% vs 30%]; no mention of ALT elevations or hepatotoxicity).
- Drugs for COPD. Med Lett Drugs Ther. 2020;62(1606):137–44. [PubMed: 32960872](Concise review of drugs used for COPD including budesonide and systemic corticosteroids indicates that they should not be used as monotherapy but rather as an add on in patients not responding to standard therapy [LABA and/or LAMA], particularly if eosinophils are greater than 300/µL; no mention of adverse events or ALT elevations or hepatotoxicity).
- Hirschfield GM, Beuers U, Kupcinskas L, Ott P, Bergquist A, Färkkilä M, Manns MP, et al. A placebo-controlled randomised trial of budesonide for primary biliary cholangitis following an insufficient response to UDCA. J Hepatol. 2020 Sep 17; Epub ahead of print. [PubMed: 32950590](Among 62 patients with primary biliary cirrhosis with an inadequate response to ursodiol who were treated with addition of budesonide vs placebo for 36 months, Alk P and ALT levels improved more with budesonide, but liver histology changes were similar in the two groups and corticosteroid related adverse events [cataracts, weight gain, hypertension, osteopenia] were more frequent with budesonide).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Exhaling a budesonide inhaler through the nose results in a significant reduction in dose requirement of budesonide nasal spray in patients having asthma with rhinitis.[J Investig Allergol Clin Immun...]Exhaling a budesonide inhaler through the nose results in a significant reduction in dose requirement of budesonide nasal spray in patients having asthma with rhinitis.Shaikh WA. J Investig Allergol Clin Immunol. 1999 Jan-Feb; 9(1):45-9.
- Effect of budesonide aqueous nasal spray on hypothalamic-pituitary-adrenal axis function in children with allergic rhinitis.[Ann Allergy Asthma Immunol. 2004]Effect of budesonide aqueous nasal spray on hypothalamic-pituitary-adrenal axis function in children with allergic rhinitis.Kim KT, Rabinovitch N, Uryniak T, Simpson B, O'Dowd L, Casty F. Ann Allergy Asthma Immunol. 2004 Jul; 93(1):61-7.
- Review Budesonide. A preliminary review of its pharmacodynamic properties and therapeutic efficacy in asthma and rhinitis.[Drugs. 1984]Review Budesonide. A preliminary review of its pharmacodynamic properties and therapeutic efficacy in asthma and rhinitis.Clissold SP, Heel RC. Drugs. 1984 Dec; 28(6):485-518.
- Review Budesonide inhalation suspension: a review of its use in infants, children and adults with inflammatory respiratory disorders.[Drugs. 2000]Review Budesonide inhalation suspension: a review of its use in infants, children and adults with inflammatory respiratory disorders.Hvizdos KM, Jarvis B. Drugs. 2000 Nov; 60(5):1141-78.
- A comparison of topical budesonide and oral montelukast in seasonal allergic rhinitis and asthma.[Clin Exp Allergy. 2001]A comparison of topical budesonide and oral montelukast in seasonal allergic rhinitis and asthma.Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. Clin Exp Allergy. 2001 Apr; 31(4):616-24.
- Budesonide - LiverToxBudesonide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...