NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Cariprazine is an atypical antipsychotic used in the treatment of schizophrenia and manic or mixed episodes of bipolar disorder. Cariprazine has been associated with a low rate of serum aminotransferase elevations during therapy, but it has not been linked to instances of clinically apparent acute liver injury.
Background
Cariprazine (kar ip' ra zeen) is an atypical antipsychotic which appears to act as a partial agonist of dopamine type 2 (D2) and 3 (D3) receptors. The D2 and D3 receptors have been identified as targets for therapy of schizophrenia where they appear to be overstimulated. Cariprazine also may have some degree of activity against selected serotonin receptors (5-HT1A). In short term clinical trials, cariprazine was shown to improve symptoms in patients with schizophrenia and manic or mixed episodes of bipolar I disorder. Cariprazine was approved for these indications in the United States in 2015, and indications were expanded subsequently to include depressive episodes associated with bipolar disorder and as adjunctive therapy to antidepressants for adults with major depressive disorder. Cariprazine is available in capsules of 1.5, 3, 4.5 and 6 mg under the brand name Vraylar. The recommended initial dose is 1.5 mg once daily, with subsequent dose increases based upon efficacy and tolerance to 3 to 6 mg daily. Lower doses are recommended for bipolar depression and when cariprazine is used as adjunctive therapy. Common side effects include dizziness, sedation, somnolence, nausea, weight gain, restlessness, tremor, akathisia and extrapyramidal symptoms. More serious adverse events can include cerebrovascular events such as transient ischemic attacks, particularly in the elderly with dementia, neurologic malignant syndrome, tardive dyskinesia, seizures, marked weight gain, dyslipidemia, diabetes, and orthostatic hypotension. Like many antipsychotic medications, cariprazine has a boxed warning for excessive mortality in elderly patients with dementia-related psychosis and, like many antidepressants, it also has a boxed warning for increased risk of suicidal thoughts and behaviors.
Hepatotoxicity
Serum aminotransferase elevations above 3 times the upper limit of normal occurred in 2% to 4% of patients treated with cariprazine in preregistration studies compared with 0.7% to 2% of placebo recipients. Elevations above 5 times ULN were rare <1% and no patient developed clinically apparent liver injury with jaundice or symptoms. Nevertheless, an occasional patient was withdrawn from therapy because of serum aminotransferase elevations usually arising within the first month of treatment and resolving rapidly with drug discontinuation. Since its approval and more widescale use, there have been no published cases of clinically apparent liver injury although the product label lists hepatitis as a possible adverse side effect.
Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which cariprazine causes serum aminotransferase elevations is not known, but is likely due to production of a toxic intermediate by its metabolism. Cariprazine is extensively metabolized by the liver via CYP 3A4 and is susceptible to drug-drug interactions with inhibitors or inducers of CYP 3A. Some instances of mild serum aminotransferase elevations occurring on cariprazine therapy may be due to nonalcoholic fatty liver disease caused by weight gain that generally occurs during the first 1 to 2 years of therapy.
Outcome and Management
The serum aminotransferase elevations that occur on cariprazine therapy are usually self-limited and usually do not require dose modification or discontinuation of therapy. Elevations of serum ALT or AST above 5 times the upper limit of normal or any elevations accompanied by jaundice or symptoms should prompt temporary discontinuation until the clinical course of the injury and role of the drug are better defined. Cariprazine has not been implicated in cases of acute liver failure, chronic liver injury or vanishing bile duct syndrome. There is no evidence to suggest cross sensitivity to liver injury between cariprazine and other atypical antipsychotic agents.
Drug Class: Antipsychotic Agents, Atypicals
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Cariprazine – Vraylar®
DRUG CLASS
Antipsychotic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
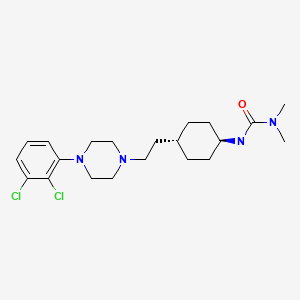
| Cariprazine | 839712-12-8 | C21-H32-Cl2-N4-O |
|
ANNOTATED BIBLIOGRAPHY
References updated: 06 June 2023
- Meyer JM. Pharmacotherapy of psychosis and mania. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 279-302.(Textbook of pharmacology and therapeutics).
- FDA. : https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2015/204370Orig1Orig2s000MedR.pdf. (FDA website with product labels and initial medical review of data presented in support of its approval as therapy of schizophrenia; mentions that ALT or AST elevations arose in 1.7-3.1% of patients treated with cariprazine vs 0.7-2.1% of placebo recipients, but elevations were transient and not accompanied by symptoms or jaundice and one patient with preexisting ALT elevations was withdrawn from therapy when ALT arose to above 10 times ULN, but bilirubin and alkaline phosphatase remained normal and ALT levels returned to baseline after stopping). - Durgam S, Starace A, Li D, Migliore R, Ruth A, Németh G, Laszlovszky I. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152:450–7. [PubMed: 24412468](Among 732 adults with an acute exacerbation of schizophrenia treated with cariprazine [1.5, 3.0 and 4.5 mg] or risperidone [4 mg] or placebo daily for 6 weeks, improvement in symptom scores occurred in all treatment groups, and side effects that were more frequent with cariprazine than placebo were insomnia, extrapyramidal symptoms, akathisia, sedation, nausea, dizziness and constipation, while mean levels of ALT increased slightly [0.8 to 3.2 U/L] in a dose dependent manner with cariprazine, however there were no cases of clinically apparent liver injury).
- Durgam S, Cutler AJ, Lu K, Migliore R, Ruth A, Laszlovszky I, Németh G, et al. Cariprazine in acute exacerbation of schizophrenia: a fixed-dose, phase 3, randomized, double-blind, placebo- and active-controlled trial. J Clin Psychiatry. 2015;76:e1574–82. [PubMed: 26717533](Among 617 patients with schizophrenia treated with cariprazine [3 or 6 mg], aripiprazole [10 mg] or placebo daily for 6 weeks, symptoms improved with cariprazine and aripiprazole compared to placebo, and side effects included insomnia, akathisia and headache).
- Calabrese JR, Keck PE Jr, Starace A, Lu K, Ruth A, Laszlovszky I, Németh G, et al. Efficacy and safety of low- and high-dose cariprazine in acute and mixed mania associated with bipolar I disorder: a double-blind, placebo-controlled study. J Clin Psychiatry. 2015;76:284–92. [PubMed: 25562205](Among 497 patients with acute and mixed mania due to bipolar I disorder treated with cariprazine or placebo for 3 weeks, mean serum ALT levels rose slightly [4-5 U/L vs 1 U/L with placebo], but mean AST, alkaline phosphatase or bilirubin values did not).
- Kane JM, Zukin S, Wang Y, Lu K, Ruth A, Nagy K, Laszlovszky I, et al. Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, phase III clinical trial. J Clin Psychopharmacol. 2015;35:367–73. [PubMed: 26075487](Among 446 patients with an acute exacerbation of schizophrenia treated with cariprazine [3-9 mg] or placebo daily for 6 weeks, symptoms improved with cariprazine therapy and adverse events included akathisia, extrapyramidal symptoms and tremor, while ALT elevations above 3 times ULN occurred in 1% of all 3 groups, but led to discontinuation in 1 cariprazine treated subject).
- Sachs GS, Greenberg WM, Starace A, Lu K, Ruth A, Laszlovszky I, Németh G, et al. Cariprazine in the treatment of acute mania in bipolar I disorder: a double-blind, placebo-controlled, phase III trial. J Affect Disord. 2015;174:296–302. [PubMed: 25532076](Among 312 patients with acute manic or mixed episodes due to bipolar I disorder treated with cariprazine [3-12 mg daily] or placebo for 3 weeks, symptom scores improved more with cariprazine while side effects included extrapyramidal symptoms, akathisia, tremor, dyspepsia and vomiting and mean changes in ALT levels were 13 vs 7 U/L; no mention of hepatotoxicity or liver related reason for discontinuation or dose modification).
- McCormack PL. Cariprazine: First global approval. Drugs. 2015;75:2035–43. [PubMed: 26510944](Review of the mechanism of action, pharmacology, clinical efficacy and safety of cariprazine as therapy for schizophrenia and manic or mixed episodes of bipolar I disorder; mentions that cariprazine has been associated with a higher rate of liver enzyme elevations compared to placebo, but that no clinically apparent liver injury with jaundice occurred).
- Durgam S, Earley W, Lipschitz A, Guo H, Laszlovszky I, Németh G, Vieta E, et al. An 8-week randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of cariprazine in patients with bipolar I depression. Am J Psychiatry. 2016;173:271–81. [PubMed: 26541814](Among 571 patients with acute manic or mixed episodes due to bipolar I disorder treated with cariprazine [0.75, 1.5 and 3 mg daily] or placebo for 6 weeks, symptom scores improved more with cariprazine and side effects included akathisia, insomnia and weight gain; there were minimal changes in serum ALT and AST values and no instance of clinically apparent liver injury with jaundice).
- Durgam S, Earley W, Li R, Li D, Lu K, Laszlovszky I, Fleischhacker WW, et al. Long-term cariprazine treatment for the prevention of relapse in patients with schizophrenia: A randomized, double-blind, placebo-controlled trial. Schizophr Res. 2016;176:264–71. [PubMed: 27427558](Among 200 patients with schizophrenia enrolled in an open label treatment study who were continued on cariprazine or placebo for up to 97 weeks, relapses occurred in 25% of cariprazine vs 48% of placebo recipients and there were slight increases in mean ALT levels, one patient developing ALT elevations and jaundice during cariprazine therapy).
- Cariprazine (Vraylar) for schizophrenia and bipolar I disorder. Med Lett Drugs Ther. 2016;58(1493):51–3. [PubMed: 27101209](Concise review of the mechanism of action, clinical efficacy, safety and costs of cariprazine shortly after its approval for use in the US; mentions side effects of extrapyramidal symptoms, akathisia, dyspepsia, vomiting, somnolence, restlessness and weight gain, but not ALT elevations or hepatotoxicity).
- Drugs for psychotic disorders. Med Lett Drugs Ther. 2016;58(1510):160–4. [PubMed: 27960194](Concise review of medications available in the US for therapy of psychotic disorders; mentions that olanzapine can cause aminotransferase elevations, and that olanzapine and ziprasidone can cause DRESS syndrome, but does not mention ALT elevations or hepatotoxicity for any of agents discussed, including aripiprazole, brexpiprazole, cariprazine, clozapine, quetiapine, risperidone, asenapine, iloperidone, paliperidone and lurasidone).
- Németh G, Laszlovszky I, Czobor P, Szalai E, Szatmári B, Harsányi J, Barabássy Á, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet. 2017;389(10074):1103–13. [PubMed: 28185672](Among 461 adults with stable schizophrenia treated with cariprazine [3-6 mg] or risperidone [3-6 mg] daily for 26 weeks, symptom scores improved more with cariprazine and rates of adverse events were similar, mean ALT and AST levels changing minimally in both groups).
- Earley W, Durgam S, Lu K, Debelle M, Laszlovszky I, Vieta E, Yatham LN. Tolerability of cariprazine in the treatment of acute bipolar I mania: A pooled post hoc analysis of 3 phase II/III studies. J Affect Disord. 2017;215:205–12. [PubMed: 28343051](Among 1065 patients with acute mania or mixed episode of bipolar I disorder treated with cariprazine or placebo for 3 weeks, adverse events more frequent with active treatment included akathisia, extrapyramidal symptoms, restlessness and vomiting; and while mean changes in ALT and AST were slightly higher with higher doses of cariprazine, there were no instances of clinically apparent liver injury with jaundice).
- Durgam S, Greenberg WM, Li D, Lu K, Laszlovszky I, Nemeth G, Migliore R, et al. Safety and tolerability of cariprazine in the long-term treatment of schizophrenia: results from a 48-week, single-arm, open-label extension study. Psychopharmacology (Berl). 2017;234:199–209. [PMC free article: PMC5203812] [PubMed: 27807604](Among 93 patients who had a response during a 6 week controlled trial and were continued on cariprazine [1.5 to 4.5 mg/day] for another 48 weeks, side effects included akathisia, insomnia and weight gain and 13% of patients had a serious adverse event, but there were no “clinically meaningful changes from baseline” in liver tests and no discontinuation was attributed to liver injury).
- Fava M, Durgam S, Earley W, Lu K, Hayes R, Laszlovszky I, Németh G. Efficacy of adjunctive low-dose cariprazine in major depressive disorder: a randomized, double-blind, placebo-controlled trial. Int Clin Psychopharmacol. 2018;33:312–321. [PMC free article: PMC6166709] [PubMed: 30045066](Among 231 patients with major depressive disorder with an inadequate response to antidepressant who were treated with addition of low or high dose cariprazine or placebo, there were numerically greater mean decreases in depression rating scores with the higher dose of cariprazine, and while adverse events were more frequent with cariprazine, mean changes in laboratory test results were “generally comparable across treatment groups” and there were no hepatic serious adverse events).
- Earley W, Burgess MV, Rekeda L, Dickinson R, Szatmári B, Németh G, McIntyre RS, et al. Cariprazine treatment of bipolar depression: a randomized double-blind placebo-controlled phase 3 study. Am J Psychiatry. 2019;176:439–448. [PubMed: 30845817](Among 480 adults with bipolar depression treated with cariprazine [1.5 or 3.0 mg] or placebo once daily for 6 weeks, clinical depression scales improved more with cariprazine [-15.1 and -15.6 vs -12.6], while side effects that were more frequent with therapy included nausea, akathisia, dizziness, sedation, and weight gain, but changes in laboratory tests were “generally comparable among treatment groups”).
- Earley WR, Burgess MV, Khan B, Rekeda L, Suppes T, Tohen M, Calabrese JR. Efficacy and safety of cariprazine in bipolar I depression: A double-blind, placebo-controlled phase 3 study. Bipolar Disord. 2020;22:372–384. [PMC free article: PMC7318333] [PubMed: 31628698](Among 493 adults with bipolar depression treated with cariprazine [1.5 or 3.0 mg daily] or placebo for 6 weeks, clinical depression scores improved more with cariprazine [-14.8 and -14.1 vs -12.4] but was significant only for the lower dose, while adverse events included nausea, fatigue, akathisia, and restlessness, but changes in laboratory values were comparable across treatment groups and there were no cases of clinically apparent liver injury or hepatic serious adverse events).
- Yatham LN, Vieta E, Earley W. Evaluation of cariprazine in the treatment of bipolar I and II depression: a randomized, double-blind, placebo-controlled, phase 2 trial. Int Clin Psychopharmacol. 2020;35:147–156. [PMC free article: PMC7099842] [PubMed: 32058426](Among 233 patients with bipolar depression treated with low or high doses of cariprazine or placebo for 6 weeks, there were no differences in improvements in depression scores in the 3 groups, but adverse events were more frequent with cariprazine including akathisia, nausea, somnolence, and weight gain, while changes in laboratory values including serum ALT and AST were similar among the 3 groups).
- Earley WR, Burgess M, Rekeda L, Hankinson A, McIntyre RS, Suppes T, Calabrese JR, et al. A pooled post hoc analysis evaluating the safety and tolerability of cariprazine in bipolar depression. J Affect Disord. 2020;263:386–395. [PubMed: 31969269](Among 2745 patients with bipolar depression enrolled in 6 clinical trials of cariprazine, adverse events were slightly more frequent with cariprazine than placebo [60% vs 55%], but there were few and only slight changes in laboratory values and no episodes of clinically apparent liver injury with jaundice).
- Heck J, Seifert J, Stichtenoth DO, Schroeder C, Groh A, Szycik GR, Degner D, et al. A case series of serious and unexpected adverse drug reactions under treatment with cariprazine. Clin Case Rep. 2021;9:e04084. [PMC free article: PMC8142394] [PubMed: 34084502](Four case reports of severe, unexpected adverse reactions to cariprazine therapy including severe akathisia, parkinsonism and hypokinesia, acute psychosis, and hyperprolactinemia; no mention of ALT elevations or hepatotoxicity).
- Barabássy Á, Sebe B, Acsai K, Laszlovszky I, Szatmári B, Earley WR, Németh G. Safety and tolerability of cariprazine in patients with schizophrenia: a pooled analysis of eight phase II/III studies. Neuropsychiatr Dis Treat. 2021;17:957–970. [PMC free article: PMC8040316] [PubMed: 33854317](In a pooled analysis based upon 2048 patients enrolled in 8 clinical trials of cariprazine for schizophrenia, the most frequent adverse events were akathisia [15%], insomnia [14%], headache [12%], and weight gain [5%] and there was a “small increase” in ALT and AST levels relative to placebo [~4 U/L]; no mention of episodes of marked ALT elevations or hepatotoxicity).
- Zeiss R, Hafner S, Schönfeldt-Lecuona C, Connemann BJ, Gahr M. Drug-associated liver injury related to antipsychotics: exploratory analysis of pharmacovigilance data. J Clin Psychopharmacol. 2022;42:440–444. [PubMed: 35730552](Review of the VigiBase data base of individual case safety reports on antipsychotics and liver injury found positive hepatic safety signals for olanzapine and clozapine, but none for risperidone, quetiapine, ziprasidone, asenapine, aripiprazole, brexpiprazole, and cariprazine).
- Cariprazine (Vraylar) for adjunctive treatment of depression. Med Lett Drugs Ther. 2023;65:84–86. [PubMed: 37216200](Concise review of the mechanism of action, clinical efficacy, safety, and cost of cariprazine as an adjunctive therapy for major depression resistant to conventional antidepressants; no mention of ALT elevations or hepatotoxicity).
- Sachs GS, Yeung PP, Rekeda L, Khan A, Adams JL, Fava M. Adjunctive cariprazine for the treatment of patients with major depressive disorder: a randomized, double-blind, placebo-controlled phase 3 study. Am J Psychiatry. 2023;180:241–251. [PubMed: 36789515](Among 751 patients with refractory major depressive disorder treated with cariprazine [1.5 or 3.0 mg] or placebo once daily for 6 weeks, depression rating scores improved more with cariprazine [-14.1 and -13.0 vs -11.5], while remission rates were similar and adverse events included akathisia, nausea, insomnia, somnolence and weight gain, but laboratory values were “generally comparable” among groups; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Asenapine.[LiverTox: Clinical and Researc...]Review Asenapine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Pharmacokinetics, Safety, and Tolerability of Cariprazine in Pediatric Patients with Bipolar I Disorder or Schizophrenia.[J Child Adolesc Psychopharmaco...]Pharmacokinetics, Safety, and Tolerability of Cariprazine in Pediatric Patients with Bipolar I Disorder or Schizophrenia.Riccobene T, Riesenberg R, Yeung PP, Earley WR, Hankinson AL. J Child Adolesc Psychopharmacol. 2022 Oct; 32(8):434-443.
- Review Cariprazine for Schizophrenia and Bipolar Disorder.[Innov Clin Neurosci. 2016]Review Cariprazine for Schizophrenia and Bipolar Disorder.Scarff JR. Innov Clin Neurosci. 2016 Sep-Oct; 13(9-10):49-52. Epub 2016 Oct 1.
- The efficacy and tolerability of cariprazine in acute mania associated with bipolar I disorder: a phase II trial.[Bipolar Disord. 2015]The efficacy and tolerability of cariprazine in acute mania associated with bipolar I disorder: a phase II trial.Durgam S, Starace A, Li D, Migliore R, Ruth A, Németh G, Laszlovszky I. Bipolar Disord. 2015 Feb; 17(1):63-75. Epub 2014 Jul 24.
- Review Mini Review on Cariprazine: A Promising Antipsychotic Agent.[CNS Neurol Disord Drug Targets...]Review Mini Review on Cariprazine: A Promising Antipsychotic Agent.Patel A, Patel A, Patel D, Patel K, Bambharoliya T. CNS Neurol Disord Drug Targets. 2023; 22(2):226-236.
- Cariprazine - LiverToxCariprazine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...