NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Clonidine is a centrally active alpha-adrenergic agonist used predominantly as an antihypertensive agent, usually in combination with other agents. Despite wide scale use for many years, clonidine has not been linked definitively to either serum aminotransferase elevations or clinically apparent liver injury.
Background
Clonidine (klon' i deen) is an antihypertensive agent which acts by stimulation of the alpha 2A subtype of alpha adrenergic receptors in the brainstem, causing a reduction in the sympathic outflow of the central nervous system. Decreases in plasma levels of norepinephrine correlate closely with its antihypertensive effects. Clonidine is effective in lowering blood pressure and can be used alone or in combination with other antihypertensive medications. Clonidine is also used in several other conditions in which central nervous system sympathetic activity is believed to contribute including neuropathy, smoking and alcohol cessation, attention deficit disorder, vascular headache, menopausal symptoms, diabetic diarrhea, and restless leg syndrome. Clonidine was approved for use in the United States in 1974 and continues to be widely used with more than 11 million prescriptions being filled yearly. Current approved indications are for treatment of hypertension, but some preparations are also approved for cancer pain management and treatment of attention deficit disorder. Off label uses include treatment of Tourette syndrome, migraine headaches, stress and sleep disorders, and to alleviate symptoms of alcohol, nicotine or narcotic withdrawal. Clonidine is available in tablets of 0.1, 0.2 and 0.3 mg generically and under the brand name of Catapres. Clonidine is also available in fixed combination with diuretics, as a solution for injection and in a transdermal formulation for application once weekly. The typical maintenance dose of clonidine in adults is 0.1 to 0.6 mg daily in 2 to 3 divided doses. Side effects are usually mild and include sedation, fatigue, bradycardia, dry mouth, headaches, dizziness, postural hypotension, male impotence and gastrointestinal upset. Sudden withdrawal can cause rebound hypertension.
Hepatotoxicity
Serum aminotransferase elevations during clonidine therapy are uncommon and rates of such elevations have not been reported in the large clinical trials, demonstrating its efficacy in hypertension. Despite many decades of use, clonidine has not been implicated in instances of clinically apparent acute liver injury.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Clonidine is metabolized by the cytochrome P450 (CYP) system to a major degree but is typically administered in very low doses (less than 1 mg daily) and neither induces or inhibits CYP activity, which may account in part for its relative lack of hepatotoxicity.
Drug Class: Antihypertensive Agents, ADHD Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Clonidine – Generic, Catapres®
DRUG CLASS
Antihypertensive Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Clonidine | 4205-90-7 | C9-H9-Cl2-N3 |
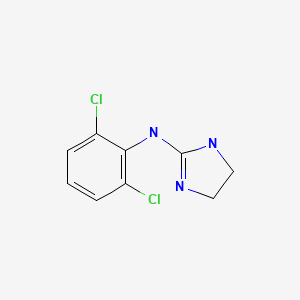
|
ANNOTATED BIBLIOGRAPHY
References updated: 25 August 2021
- Zimmerman HJ. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 639-71.(Expert review of hepatotoxicity published in 1999; clonidine is not discussed).
- Bhardwaj SS, Chalasani NP. Antihypertensive agents: Hepatotoxicity of cardiovascular and antidiabetic medications. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 2nd ed. New York: Informa Healthcare USA, 2007, pp. 603.(Review of hepatotoxicity of hypertensive agents published in 2007; “clonidine does not seem to cause hepatotoxicity”).
- Michel T, Hoffman BB. Therapy of myocardial ischemia and hypertension. In, Brunton LL, Lazo JS, Parker KL, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 745-88.(Textbook of pharmacology and therapeutics; clonidine, guanabenz, and guanfacine stimulate the alpha 2A subtype of the alpha adrenergic receptors in the brainstem and result in reduction in sympathetic outflow from the CNS).
- Ferder L, Inserra F, Medina F. Safety aspects of long-term antihypertensive therapy (10 years) with clonidine. J Cardiovasc Pharmacol. 1987;10 Suppl 12:S104–8. [PubMed: 2455159](Among 128 patients with hypertension treated with clonidine for up to 10 years, common side effects were drowsiness, dry mouth, constipation, dizziness, postural hypotension and male impotence; there were no significant changes in ALT, AST or Alk P and no discontinuations for liver test abnormalities).
- Berg PA. Dtsch Med Wochenschr. 1993;118:1339–40. [Clonidine therapy as cause of visceral lupus erythematosus?] German. [PubMed: 8375310](83 year old woman on long term clonidine for hypertension and recent addition of enalapril and HCTZ developed fatigue, dry mouth and elevated Alk P and ANA positivity [few details given], which was interpreted as possibly due to clonidine).
- Heilmann G, Hien P. Dtsch Med Wochenschr. 1994;119:858–9. [Clonidine therapy as cause of lupus erythematosus] German. [PubMed: 8005062](Discussion of the likelihood that ACE inhibitors rather than clonidine were the cause of a lupus-like syndrome with hepatotoxicity discussed by Berg [1993]).
- Drugs for hypertension. Treat Guidel Med Lett. 2009;7:1–10. [PubMed: 19107095](Brief overview of currently available drugs for hypertension with guidelines on their use and information on prices and toxicities; “Drugs such as clonidine, guanabenz, guanfacine and methyldopa decrease sympathetic outflow… and frequently cause sedation, dry mouth and erectile dysfunction”).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 39 cases [4%] were due to antihypertensive medications, but none to clonidine).
- Drugs for hypertension. Med Lett Drugs Ther. 2017;59(1516):41–8. [PubMed: 28263286](Brief overview of currently available drugs for hypertension with guidelines on their use and information on prices and toxicities; clonidine is listed as a central alpha-adrenergic agonist whose most frequent side effects are dry mouth, sedation, heart block, bradycardia and rebound hypertension; no mention of ALT elevations or hepatotoxicity).
- Padilha SCOS, Virtuoso S, Tonin FS, Borba HHL, Pontarolo R. Efficacy and safety of drugs for attention deficit hyperactivity disorder in children and adolescents: a network meta-analysis. Eur Child Adolesc Psychiatry. 2018;27:1335–45. [PubMed: 29460165](Systematic review of efficacy and safety of drugs for ADHD based upon 48 trials [4169 participants]; makes no mention of ALT elevations or hepatotoxicity of any of the agents studied, including guanfacine).
- Cortese S, Adamo N, Mohr-Jensen C, Hayes AJ, Bhatti S, Carucci S, Del Giovane C, et al. European ADHD Guidelines Group (EAGG). Comparative efficacy and tolerability of pharmacological interventions for attention-deficit/hyperactivity disorder in children, adolescents and adults: protocol for a systematic review and network meta-analysis. BMJ Open. 2017;7:e013967. [PMC free article: PMC5253538] [PubMed: 28073796](Review of the pharmacological therapy of ADHD with discussion of amphetamines, methylphenidate, atomoxetine, guanfacine and clonidine; no mention of ALT elevations during therapy or hepatotoxicity).
- Schoretsanitis G, de Leon J, Eap CB, Kane JM, Paulzen M. Clinically significant drug-drug interactions with agents for attention-deficit/hyperactivity disorder. CNS Drugs. 2019;33:1201–1222. [PubMed: 31776871](Review of drug-drug interactions of agents used to treat ADHD mentions that there is little information on the metabolism of clonidine and possible drug-drug interactions).
- Drugs for ADHD. Med Lett Drugs Ther. 2020;62(1590):9–15. [PubMed: 31999670](Concise review of mechanism of action, clinical efficacy, safety and cots of drugs for ADHD mentions that clonidine is a nonstimulatory agent that is generally well tolerated; no mention of ALT elevations or hepatotoxicity).
- Drugs for hypertension. Med Lett Drugs Ther. 2020;62(1598):73–80. [PubMed: 32555118](Brief overview of currently available drugs for hypertension with guidelines on their use and information on prices and toxicities; clonidine is listed as a central alpha-adrenergic agonist used mostly as add-on treatment in patients with refractory hypertension, which can cause sedation, dry mouth, and erectile dysfunction; no mention of ALT elevations or hepatotoxicity).
- Burek GA, Waite MR, Heslin K, Liewen AK, Yaqub TM, Larsen SE. Low-dose clonidine in veterans with Posttraumatic stress disorder. J Psychiatr Res. 2021;137:480–485. [PubMed: 33798975](Retrospective analysis of 79 male, military veterans with severe post-traumatic stress disorder [PTSD] who were treated with low doses of clonidine [0.05 to 0.5 mg daily] in whom mean Clinical Global Impression scores improved from 4.8 to 2.9, and 23% of whom reported adverse events which were mostly mild, with no serious adverse events and no mention of hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Role of medullary I1-imidazoline and alpha 2-adrenergic receptors in the antihypertensive responses evoked by central administration of clonidine analogs in conscious spontaneously hypertensive rats.[J Pharmacol Exp Ther. 1995]Role of medullary I1-imidazoline and alpha 2-adrenergic receptors in the antihypertensive responses evoked by central administration of clonidine analogs in conscious spontaneously hypertensive rats.Buccafusco JJ, Lapp CA, Westbrooks KL, Ernsberger P. J Pharmacol Exp Ther. 1995 Jun; 273(3):1162-71.
- Hypotensive and sedative effects of alpha-adrenoceptor agonists: relationship to alpha 1- and alpha 2-adrenoceptor potency.[Br J Pharmacol. 1981]Hypotensive and sedative effects of alpha-adrenoceptor agonists: relationship to alpha 1- and alpha 2-adrenoceptor potency.Clough DP, Hatton R. Br J Pharmacol. 1981 Jul; 73(3):595-604.
- Endogenous opioid peptides: do they mediate the acute antihypertensive action of clonidine in humans?[Horm Res. 1986]Endogenous opioid peptides: do they mediate the acute antihypertensive action of clonidine in humans?Levin ER, Sharp B, Weber MA, Drayer JI. Horm Res. 1986; 23(4):193-9.
- Review The hypotensive activity and side effects of methyldopa, clonidine, and guanfacine.[Hypertension. 1984]Review The hypotensive activity and side effects of methyldopa, clonidine, and guanfacine.van Zwieten PA, Thoolen MJ, Timmermans PB. Hypertension. 1984 Sep-Oct; 6(5 Pt 2):II28-33.
- Review New centrally acting antihypertensive drugs related to methyldopa and clonidine.[Hypertension. 1984]Review New centrally acting antihypertensive drugs related to methyldopa and clonidine.Sweet CS. Hypertension. 1984 Sep-Oct; 6(5 Pt 2):II51-6.
- Clonidine - LiverToxClonidine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...