NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Dolutegravir is a human immunodeficiency virus (HIV) integrase inhibitor, the third in this class of agents that target the viral integrase. Dolutegravir is used only in combination with other antiretroviral agents in the treatment of HIV infection, and it has had limited use. Dolutegravir is associated with a low rate of serum aminotransferase elevations during therapy, but has not been linked to instances of acute, clinically apparent liver injury.
Background
Dolutegravir (doe" loo teg' ra vir) is relatively new antiretroviral drug that targets the HIV integrase, one of the three viral enzymes involved in replication. Dolutegravir blocks the binding site of the HIV integrase and prevents the strand transfer activity and integration of the provirus into the host genome. Dolutegravir has both in vitro and in vivo activity against HIV, and several randomized controlled trials have shown that it can cause significant declines in HIV RNA levels and rises in peripheral CD4 T cell counts. Dolutegravir was approved for use in HIV infection in the United States in 2013 and is currently used in an increasing proportion of antiretroviral regimens. Dolutegravir is available as 50 mg tablets under the brand name Tivicay. The recommended dose regimen is 50 mg twice daily in combination with other classes of antiretroviral agents. A single tablet, fixed dose regimen of dolutegravir [50 mg], abacavir [600 mg] and lamivudine [300 mg] is also available under the name Triumeq, the recommended dose being one tablet daily. While generally well tolerated, dolutegravir therapy may be accompanied by diarrhea, headache, nausea and fever.
Hepatotoxicity
In large clinical trials, therapy with dolutegravir was associated with alanine aminotransferase (ALT) elevations of greater than 3 times the upper limit of normal (ULN) in 2% to 5% of patients, but these rates were similar to those in comparator groups receiving matched background optimized antiretroviral therapy without dolutegravir. These elevations were not associated with clinical symptoms and generally did not require dose modification. A few instances of acute liver injury with jaundice were described in the registration trials for dolutegravir which occurred in association with hypersensitivity reactions and resolved with drug discontinuation. The clinical features of these cases were not provided and their association with dolutegravir as opposed to the concurrent antiretroviral agents was not fully established. Since its approval and more wide spread use, however, several case reports of acute hepatitis attributable to dolutegravir have appeared. The latency to onset varried from 1 to 8 months and the pattern of serum enzyme elevations was hepatocellular. Immunoallergic and autoimmune features were not present. At least one published case resulted in acute liver failure and need for liver transplanation. The product label for dolutegravir mentions hepatitis and hepatic failure as potential adverse reactions and states that patients with hepatitis B or C coinfection are susceptible to worsening or flares of hepatitis with initiation of dolutegravir therapy, perhaps as a consequence of immune reconstitution syndrome. Monitoring of liver tests is recommended in patients starting regimens that include dolutegravir.
Likelihood score: D (possible cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which dolutegravir might cause liver injury is not known. Dolutegravir is metabolized by the liver, largely by glucuronidation with subsequent urinary clearance. Dolutegravir has little or no effect on microsomal cytochrome P450 enzymes. Reconstitution of immune reactivity by antiretroviral therapy in patients with an underlying chronic hepatitis B or C can be associated with a transient flare of hepatitis. This effect occurs with many potent antiretroviral regimens and is not specific to dolutegravir or the integrase inhibitors.
Outcome and Management
Dolutegravir is associated with a low rate of serum enzyme elevations during therapy and monitoring of liver tests is recommended. Cases of acute hepatitis including fatal instances have been reported. Elevations of serum aminotransferase levels above 5 times the ULN should lead to dose interruptions and permanent discontinuation if they persist or are associated with clinical symptoms or jaundice. Switching to other antiretroviral agents is appropriate iand there does not appear to be cross sensitivity to hepatic injury among the various HIV integrase inhibitors. Dolutegravir has not been linked to cases of chronic cholestasis or vanishing bile duct syndrome.
Drug Class: Antiviral Agents
Other Drugs in the Subclass, Integrase Strand Transfer Inhibitors: Bictegravir, Cabotegravir, Elvitegravir, Raltegravir
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Dolutegravir – Tivicay®
DRUG CLASS
Antiviral Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Dolutegravir | 1051375-16-6 | C20-H19-F2-N3-O5 |
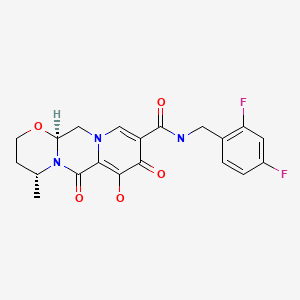
|
| Elvitegravir | 697761-98-1 | C23-H23-Cl-F-N-O5 |
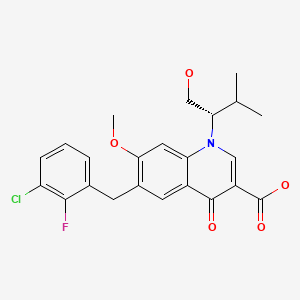
|
| Raltegravir | 518048-05-0 | C20-H21-F-N6-O5 |

|
ANNOTATED BIBLIOGRAPHY
References updated: 10 January 2018
- Núñez M. Hepatic toxicity of antiviral agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 505-18.(Review of hepatotoxicity of antiviral agents including drugs for HIV infection, although the integrase inhibitors are not discussed).
- Flexner C. Antiretroviral agents and treatment of HIV infection. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1623-64.(Textbook of pharmacology and therapeutics).
- https://hivinfo
.nih.gov /hiv-source/medical-practice-guidelines /hiv-treatment-guidelines . (Regularly updated clinical guidelines on the use of antiretroviral agents in HIV-1 infected adults, adolescents and children). - Cocohoba J, Dong BJ. Raltegravir: the first HIV integrase inhibitor. Clin Ther. 2008;30:1747–65. [PubMed: 19014832](Review of the mechanism of action, pharmacokinetics, and phase I-III studies of raltegravir; in combined phase III studies, ALT elevations above 5 times ULN occurred in 4.3% of 462 raltegravir treated vs 3.4% of 237 placebo treated patients who were receiving optimized background therapy; no hepatic serious adverse events were reported).
- Fleischbein E, O'Brien J, Martelino R, Fenstersheib M. Elevated alkaline phosphatase with raltegravir in a treatment experienced HIV patient. AIDS. 2008;22:2404–5. [PubMed: 18981785](45 year old man with AIDS developed elevated Alk P [85 rising to 1053 U/L], which resolved upon stopping and recurred on rechallenge with raltegravir; serum bilirubin, ALT and GGT levels were normal, but authors suspected liver as the source anyway).
- A 4-drug combination (Stribild) for HIV. Med Lett Drugs Ther. 2012;54(1404):95–6. [PubMed: 23183388](Concise review of the mechanism of action, clinical efficacy, safety and cost of the single table regimen of Stribild [elvitegravir, tenofovir, emtricitabine and cobicistat], shortly after its approval in the US; does not mention ALT elevations or hepatotoxicity).
- Surgers L, Lacombe K. Hepatoxicity of new antiretrovirals: a systematic review. Clin Res Hepatol Gastroenterol. 2013;37:126–33. [PubMed: 23522569](Review of evidence of liver injury in large clinical trials of newer antiretroviral agents including dolutegravir concluded that "the overall hepatic tolerance is far better with the novel drugs in this review than with former ARV regimens"; ALT elevations occurred with equal frequency in dolutegravir and comparator arms, and one patient on raltegravir developed DRESS syndrome).
- Eron JJ, Clotet B, Durant J, Katlama C, Kumar P, Lazzarin A, Poizot-Martin I, et al. VIKING Study Group. Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING Study. J Infect Dis. 2013;207:740–8. [PMC free article: PMC3563307] [PubMed: 23225901](Among 52 HIV infected persons with raltegravir resistance who were switched to dolutegravir [50 mg once or twice daily], virological responses at 11 days were more frequent with the twice daily dosing, and there were no liver related severe adverse events and no mention of ALT levels).
- Raffi F, Rachlis A, Stellbrink HJ, Hardy WD, Torti C, Orkin C, Bloch M, et al. SPRING-2 Study Group. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381:735–43. [PubMed: 23306000](Among 822 treatment naïve patients with HIV infection treated with dolutegravir vs raltegravir combined with tenofovir/emtricitabine or abacavir/lamivudine, virological response rates at 48 weeks were similar [88% vs 85%] as were adverse event rates; ALT elevations above 3 times ULN occurred in 13 [3%] vs 17 [4%] patients and above 10 times ULN in 5 [1%] vs 2 [<1%], one patient in each group having “possible” drug induced liver injury, both with signs of hypersensitivity).
- Stellbrink HJ, Reynes J, Lazzarin A, Voronin E, Pulido F, Felizarta F, Almond S, et al. SPRING-1 Team. Dolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging study. AIDS. 2013;27:1771–8. [PMC free article: PMC3694319] [PubMed: 23807273](Among 205 treatment naïve adults with HIV infection treated with one of 3 doses of dolutegravir or efavirenz once daily combined with two reverse transcriptase inhibitors, virological response rates were 78-88% with dolutegravir and 72% with efavirenz, and there were no treatment related serious adverse events; 2 patients developed ALT levels above 5 times ULN, but both were found to have acute hepatitis C).
- Cahn P, Pozniak AL, Mingrone H, Shuldyakov A, Brites C, Andrade-Villanueva JF, Richmond G, et al. extended SAILING Study Team. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382(9893):700–8. [PubMed: 23830355](Among 715 patients with HIV infection treated with either dolutegravir or raltegravir combined with background antiretroviral therapy, adverse events were similar between the two groups and 3% vs 2% developed ALT elevations above 5 times ULN; no mention of clinically apparent liver injury).
- Raffi F, Jaeger H, Quiros-Roldan E, Albrecht H, Belonosova E, Gatell JM, Baril JG, et al. extended SPRING-2 Study Group. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naïve adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2013;13:927–35. [PubMed: 24074642](Among 822 patients with previously untreated HIV infection given dolutegravir vs raltegravir in combination with other agents [Raffi Lancet 2013], 96 week virological response rates were 81% vs 76% and no new treated related, serious adverse events arose during the second 48 weeks of the trial; ALT elevations above 3 times ULN occurred in a total of 21 [5%] vs 19 [5%] subjects).
- Walmsley SL, Antela A, Clumeck N, Duiculescu D, Eberhard A, Gutiérrez F, Hocqueloux L, et al. SINGLE Investigators. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369:1807–18. [PubMed: 24195548](Among 833 treatment naïve adults with HIV infection treated with dolutegravir with abacavir/lamivudine or efavirenz with tenofovir/emtricitabine, virological response rates at 48 weeks were higher with dolutegravir [88% vs 81%] and adverse events were less common; ALT levels above 2.5 times ULN occurred in 2% vs 5% of subjects and no patient developed clinically apparent liver injury with jaundice).
- Curtis L, Nichols G, Stainsby C, Lim J, Aylott A, Wynne B, Clark A, et al. Dolutegravir: clinical and laboratory safety in integrase inhibitor-naive patients. HIV Clin Trials. 2014;15:199–208. [PubMed: 25350958](Summary of laboratory abnormalities in the registration trials of dolutegravir in adults with HIV infection found ALT elevations above 2.5 times ULN in 16% to 22% of patients with hepatitis B or C coinfection, but in only 3% with HIV infection alone; serious hypersensitivity reactions occurred only in patients who also received abacavir and no instances of severe cutaneous reactions such as Stevens Johnson syndrome occurred in dolutegravir treated subjects).
- Molina JM, Clotet B, van Lunzen J, Lazzarin A, Cavassini M, Henry K, Kulagin V, et al. FLAMINGO study team. Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomised, open-label, phase 3b study. Lancet HIV. 2015;2:e127–36. [PubMed: 26424673](Among 484 treatment naïve adults with HIV infection treated with dolutegravir or darunavir/ritonavir combined with two reverse transcriptase inhibitors, virological response rates at 96 weeks were 80% vs 68%; ALT levels above 3 times ULN occurred in 5% vs 3% of patients, but no patient developed clinically apparent liver injury with jaundice and all serious liver test abnormalities were considered to have other causes).
- Dolutegravir (Tivicay) for HIV. Med Lett Drugs Ther. 2013;55(1426):77–9. [PubMed: 24081387](Concise review of the mechanism of action, clinical efficacy, safety and cost of dolutegravir shortly after its approval for use in HIV infection in the US, mentions that it is generally well tolerated, causes hypersensitivity reactions in <1% of persons and has been associated with ALT elevations in patients with hepatitis B or C coinfection).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–1352.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 12 [1.3%] cases were attributed to antiretroviral agents, but none to the integrase inhibitors - dolutegravir, elvitegravir or raltegravir).
- Triumeq--a 3-drug combination for HIV. Med Lett Drugs Ther. 2015;57(1459):7–8. [PubMed: 25555073](Concise review of the mechanism of action, clinical efficacy, safety and cost of the fixed dose, single tablet regimen of dolutegravir, abacavir and lamivudine [Triumeq] shortly after its approval for use in HIV infection in the US, mentions that common side effects are insomnia, headache and fatigue and rare severe reactions include hypersensitivity responses with abacavir, lactic acidosis with lamivudine and abacavir and ALT elevations in patients with hepatitis B and C with dolutegravir).
- Elvitegravir (Vitekta) for HIV. Med Lett Drugs Ther. 2016;58(1486):10–1. [PubMed: 26761343](Concise review of the mechanism of action, clinical efficacy, safety and cost of elvitegravir shortly after its approval for separate use in HIV infection in the US, mentions that the most common side effects are diarrhea, nausea and headache; no mention of ALT elevations or hepatotoxicity).
- Wang B, Abbott L, Childs K, Taylor C, Agarwal K, Cormack I, Miquel R, Suddle A. Dolutegravir-induced liver injury leading to sub-acute liver failure requiring transplantation: a case report and review of literature. Int J STD AIDS. 2018;29(4):414–417. [PubMed: 29059031](28 year old African woman with HIV infection developed persistent ALT elevations a month after switching from raltegravir/ tenofovir/emtricitabine to dolutegravir/abacavir/lamivudine [ALT 300 U/L], which worsened despite switching back [bilirubin 7.3 mg/dL, ALT 1636 U/L, Alk P 131 U/L, INR 2.14], ultimately requiring liver transplantation for acute liver failure).
- Christensen ES, Jain R, Roxby AC. Abacavir/dolutegravir/lamivudine (Triumeq)-induced liver toxicity in a human immunodeficiency virus-infected patient. Open Forum Infect Dis. 2017;4:ofx122. [PMC free article: PMC5522577] [PubMed: 28748198](47 year old man with HIV developed ALT elevations 8 months after starting dolutegravir/abacavir/lamivudine [bilirubin 0.8 mg/dL, ALT 343 U/L], which worsened with continuing [bilirubin 2.1 mg/dL, ALT 1004 U/L], improving on stopping and switching to elvitegravir/tenofovir/emtricitabine).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Bictegravir.[LiverTox: Clinical and Researc...]Review Bictegravir.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- The R263K Dolutegravir Resistance-Associated Substitution Progressively Decreases HIV-1 Integration.[mBio. 2017]The R263K Dolutegravir Resistance-Associated Substitution Progressively Decreases HIV-1 Integration.Mesplède T, Leng J, Pham HT, Liang J, Quan Y, Han Y, Wainberg MA. mBio. 2017 Apr 4; 8(2). Epub 2017 Apr 4.
- Review Dolutegravir, a second-generation integrase inhibitor for the treatment of HIV-1 infection.[Ann Pharmacother. 2014]Review Dolutegravir, a second-generation integrase inhibitor for the treatment of HIV-1 infection.Rathbun RC, Lockhart SM, Miller MM, Liedtke MD. Ann Pharmacother. 2014 Mar; 48(3):395-403. Epub 2013 Nov 19.
- Integrase Inhibitor Resistance Mechanisms and Structural Characteristics in Antiretroviral Therapy-Experienced, Integrase Inhibitor-Naive Adults with HIV-1 Infection Treated with Dolutegravir plus Two Nucleoside Reverse Transcriptase Inhibitors in the DAWNING Study.[Antimicrob Agents Chemother. 2...]Integrase Inhibitor Resistance Mechanisms and Structural Characteristics in Antiretroviral Therapy-Experienced, Integrase Inhibitor-Naive Adults with HIV-1 Infection Treated with Dolutegravir plus Two Nucleoside Reverse Transcriptase Inhibitors in the DAWNING Study.Underwood M, Horton J, Nangle K, Hopking J, Smith K, Aboud M, Wynne B, Sievers J, Stewart EL, Wang R. Antimicrob Agents Chemother. 2022 Jan 18; 66(1):e0164321. Epub 2021 Oct 25.
- Dolutegravir in HIV-2-Infected Patients With Resistant Virus to First-line Integrase Inhibitors From the French Named Patient Program.[Clin Infect Dis. 2015]Dolutegravir in HIV-2-Infected Patients With Resistant Virus to First-line Integrase Inhibitors From the French Named Patient Program.Descamps D, Peytavin G, Visseaux B, Tubiana R, Damond F, Campa P, Charpentier C, Khuong-Josses MA, Duvivier C, Karmochkine M, et al. Clin Infect Dis. 2015 May 15; 60(10):1521-7. Epub 2015 Feb 17.
- Dolutegravir - LiverToxDolutegravir - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...