NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Oral means of emergency contraception, colloquially known as “morning after” pills, are used to prevent unwanted pregnancy after unprotected intercourse or suspected contraceptive failure. Two commonly used forms of emergency contraception are levonorgestrel and ulipristal acetate. Both can be administered as a single oral dose, ulipristal within 5 days and levonorgestrel within 2 days of the unprotected intercourse. Both have been shown to be safe and effective, rates of subsequent pregnancy being 0.6% to 3% compared to an expected rate of 5% to 6%. Levonorgestrel is available over-the-counter whereas ulipristal, at least in the United States, is available by prescription only. Neither approach to emergency contraception has been linked to serum enzyme elevations or to clinically apparent liver injury. However, ulipristal when given in daily doses in 3 month courses as therapy for symptomatic uterine fibroids has been linked to several instances of clinically apparent liver injury, some of which have been severe and even fatal.
Levonorgestrel
Introduction
Levonorgestrel is a synthetic progesterone that is used for emergency contraception. Levonorgestrel is also used alone and in combination with estrogens in conventional oral contraceptives. Use of levonorgestrel for emergency contraception has not been associated with serum enzyme elevations or clinically apparent liver injury with jaundice.
Background
Levonorgestrel (lee" voe nor jes' trel) is a synthetic progesterone that is used as a single or as two oral doses within 2 days of unprotected intercourse or contraceptive failure as a means of emergency contraception. Levonorgestrel acts as an agonist of the progesterone receptor and is believed to act by preventing ovulation. In both animal models and in human trials, levonorgestrel appears to be effective only when given before the luteinizing hormone (LH) surge that precedes ovulation and has little or no effect on fertilization or implantation. In large prospective studies, levonorgestrel given in a single or as two doses 12 hours apart lowered the rate of unwanted pregnancies by 50% to 90% of the expected rate, and the effect was seen for as long as 48 hours after unprotected intercourse or suspected contraceptive failure. The overall failure rates of levonorgestrel have ranged from 0.6% to 3.1%, the expected rates being 5% to 6%. Levonorgestrel was approved in the United States for use as emergency contraception in 1982 and remains available generically and over-the-counter as tablets of 0.75 and 1.5 mg. The recommended regimen is a total dose of 1.5 mg within 72 hours of the unprotected intercourse or suspected contraceptive failure, given either as a single or split (12 hours apart) oral dose. Side effects are generally mild-to-moderate in severity and can include headache, nausea, abdominal pain, dysmenorrhea, breast tenderness, fatigue, and dizziness.
Levonorgestrel is also used as a progestin in conventional oral contraceptives, administered alone or in combination with estrogens. The safety and hepatotoxicity of oral contraceptives are discussed separately in LiverTox in sections Estrogens and Oral Contraceptives, and Progesterone and the Progestins.
Hepatotoxicity
In large prospective trials of levonorgestrel as emergency contraception that included laboratory testing, mean serum ALT and AST levels remained unchanged and only rare, minor elevations of serum enzymes occurred that were invariably transient, not accompanied by jaundice or symptoms and not considered related to the emergency contraception. Despite widescale use of levonorgestrel for several decades, there have been no published reports of hepatotoxicity attributable to its use as emergency contraception.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which levonorgestrel used for emergency contraception might cause liver injury is uncertain. It is typically given in one or two low doses, the total amount being only 1.5 mg. Levonorgestrel is metabolized in the liver predominantly via CYP 3A4 and is susceptible to drug-drug interactions with potent inducers or inhibitors of the drug-metabolizing enzyme. Levonorgestrel is a progesterone, but has not been linked to the cholestatic forms of injury associated with convention oral contraceptives that contain progesterone alone or combined with estrogens.
Ulipristal
Introduction
Ulipristal acetate is a selective progesterone receptor modulator that is used in a single oral dose for emergency contraception. Use of ulipristal has not been associated with serum enzyme elevations or with clinically apparent liver injury with jaundice.
Background
Ulipristal (ue" le pris' tal) is a selective progesterone receptor modulator that is used in a single oral dose within 5 days of unprotected intercourse or suspected contraceptive failure as a means of emergency contraception. Ulipristal acts as a mixed, partial agonist and antagonist of the progesterone receptor and is believed to act by preventing both ovulation and fertilization. Unlike levonorgestrel, ulipristal can be used up to 5 days after the episode of unprotected intercourse, being effective both before and during the early phase of the luteinizing hormone (LH) surge. Ulipristal has minor effects on the uterine epithelium that may inhibit implantation. In large prospective studies, ulipristal was found to lower the rate of unwanted pregnancies by 50% to 90% of the expected rate when given up to 5 days after unprotected intercourse or suspected contraceptive failure. The overall failure rate of emergency contraception with ulipristal has ranged from 0.9% to 2.1%, the expected rates being in the range of 5% to 6%. Ulipristal was approved in the United States for use in emergency contraception in 2010 and remains available by prescription as tablets of 30 mg under the brand name Ella. The recommended regimen is a single oral dose of 30 mg within 5 days of the unprotected intercourse or suspected contraceptive failure. Side effects are generally mild-to-moderate in severity and can include headache, nausea, abdominal pain, dysmenorrhea, breast tenderness, fatigue, and dizziness.
Ulipristal has also been evaluated as nonsurgical therapy of uterine fibroids. When given in daily doses of 5 to 10 mg for 2 to 3 months, ulipristal was found to decrease uterine bleeding and total fibroid volume. Side effects included amenorrhea, headaches, hot flushes, nausea, vomiting, abdominal cramps and menorrhagia. Ulipristal was approved for this indication in the European Union, but its use was restricted after several reports of severe liver injury were reported in women being treated for uterine fibroids.
Hepatotoxicity
In large prospective trials of ulipristal that included laboratory testing, there were isolated moderate elevations in ALT levels, but all were transient, less than 3 times the upper limit of normal and not accompanied by jaundice or symptoms. Since approval of ulipristal in 2010 there have been no reports of liver injury associated with its use in emergency contraception as a one-time dose.
Likelihood score when used for emergency contraception: E (unlikely cause of clinically apparent liver injury).
Ulipristal has also been used in daily doses of 5 mg in 3 month courses as therapy of uterine fibroids. In preregistration trials, ulipristal when given in doses of 5 mg daily was not associated with serum enzyme elevations or with instances of clinically apparent liver injury. Ulipristal did not receive approval as therapy of uterine fibroids in the United States but was approved for this indication in the European Union in 2012. Subsequently, there have been several reports of severe liver injury, including acute liver failure requiring liver transplantation in women being treated for fibroids. The latency to onset ranged from 1 to 6 months and the clinical presentation was with fatigue and an acute hepatocellular pattern of enzyme elevations followed by jaundice. Several instances of acute liver failure were reported to the European Medicines Agency which conducted a formal review of the hepatotoxicity of ulipristal in 2018, which led to a restriction in its use to moderately-to-severely symptomatic uterine fibroids unresponsive to other therapies. Avoidance of therapy in patients with preexisting liver disease and monthly monitoring for serum enzyme elevations was also recommended, with prompt discontinuation for de novo elevations. Whether prospective monitoring is effective in preventing serious liver injury in women treated with ulipristal is unclear. Similar cases of clinically apparent liver injury have not been reported with the use of ulipristal as an emergency contraceptive agent.
Likelihood score when used in daily doses for uterine fibroids: C (probable cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which ulipristal might cause liver injury is uncertain. It is typically given as a single dose for emergency contraception, but in daily somewhat low doses for symptomatic uterine fibroids. Ulipristal is metabolized in the liver predominantly via CYP 3A4 and is susceptible to drug-drug interactions with potent inducers or inhibitors of CYP 3A. Ulipristal is a partial progesterone receptor agonist, but has not been linked to the cholestatic forms of injury associated with estrogen and progesterone administration. The hepatocellular injury associated with long term use appears to be idiosyncratic.
Drug Class: Obstetrical and Gynecological Agents, Emergency Contraceptive Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Levonorgestrel – Generic, Plan B®
Ulipristal Acetate – Ella®
DRUG CLASS
Emergency Contraceptive Agents
COMPLETE LABELING
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Levonorgestrel | 797-63-7 | C21-H28-O2 |
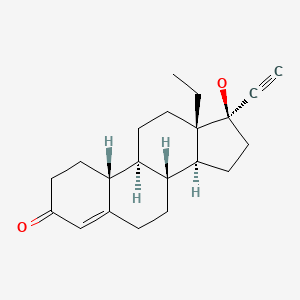
|
| Ulipristal | 159811-51-5 | C28-H35-N-O3 |
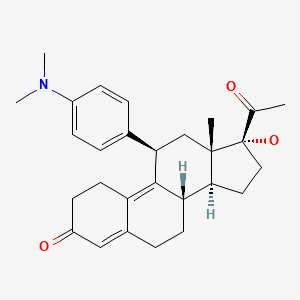
|
| Progesterone | 57-83-0 | C21-H30-O2 |
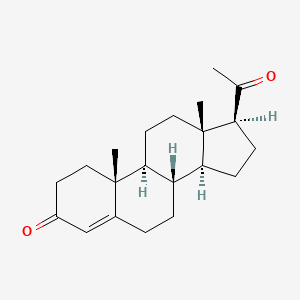
|
ANNOTATED BIBLIOGRAPHY
References updated: 02 June 2020
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999; does not specifically discuss levonorgestrel or ulipristal).
- Chitturi S, Farrell GC. Estrogen receptor antagonists. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 610-2.(Review of hepatotoxicity of hormonal agents; discusses estrogens and progestins, but does not specifically discuss levonorgestrel or ulipristal).
- Schimmer BP, Parker KL. Contraception and pharmacotherapy of obstetrical and gynecological disorders. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1833-52.(Textbook of pharmacology and therapeutics).
- Emergency contraception OTC. Med Lett Drugs Ther. 2004;46(1175):10–1. [PubMed: 14749610](Concise review of the efficacy, safety and costs of the over-the-counter form of Plan B that contains two 0.75 mg tablets of levonorgestrel; mentions side effects of nausea and vomiting, headache, abdominal pain and breast tenderness).
- Glasier AF, Cameron ST, Fine PM, Logan SJ, Casale W, Van Horn J, Sogor L, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375(9714):555–62. [PubMed: 20116841](Among 1899 women requesting emergency contraception within 5 days of unprotected sexual intercourse who were treated with either ulipristal [30 mg] or levonorgestrel [1.5 mg] and had adequate follow up evaluations, 50 pregnancies occurred and rates were lower with ulipristal [1.8% vs 2.6%] and adverse events were similar in both groups; no mention of ALT elevations or hepatotoxicity).
- Ella: a new emergency contraceptive. Med Lett Drugs Ther. 2011;53(1355):3–4. [PubMed: 21212744](Concise review of the mechanism of action, clinical efficacy, safety, and costs of ulipristal acetate as emergency contraception shortly after its approval for use in the US; mentions its side effects of headache, nausea, abdominal pain, dysmenorrhea, fatigue, and dizziness, but not serious adverse events, ALT elevations or hepatotoxicity).
- Snow SE, Melillo SN, Jarvis CI. Ulipristal acetate for emergency contraception. Ann Pharmacother. 2011;45:780–6. [PubMed: 21666089](Review of the safety and efficacy of ulipristal for emergency contraception; mentions that common adverse events included headache [17%], breast tenderness [16%], nausea [15%] and abdominal pain [13%], and that severe adverse events were rare and none were considered related to ulipristal).
- Chen QJ, Xiang WP, Zhang DK, Wang RP, Luo YF, Kang JZ, Cheng LN. Efficacy and safety of a levonorgestrel enteric-coated tablet as an over-the-counter drug for emergency contraception: a Phase IV clinical trial. Hum Reprod. 2011;26:2316–21. [PMC free article: PMC3157624] [PubMed: 21672924](Among 2445 women requesting emergency contraception treated with levonorgestrel within 72 hours of unprotected intercourse, the pregnancy rate was 0.2% and adverse events included nausea, vomiting, vaginal bleeding, headache, and dizziness, but there was no mention of ALT elevations or liver related adverse events).
- Cheng L, Che Y, Gülmezoglu AM. Interventions for emergency contraception. Cochrane Database Syst Rev. 2012;(8):CD001324. [PubMed: 22895920](Systematic analysis of 100 randomized controlled trials of emergency contraception with 55,666 women using mifepristone, levonorgestrel, copper intrauterine device placement and ulipristal, reported that all regimens were safe; no mention of ALT elevations or hepatotoxicity).
- Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, Ugocsai G, et al. PEARL I Study Group. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409–20. [PubMed: 22296075](Among 239 women with symptomatic uterine fibroids and excessive bleeding treated with ulipristal [5 vs 10 mg] or placebo daily for up to 13 weeks before surgery, uterine bleeding and fibroid size decreased with ulipristal and adverse events included amenorrhea [73% and 82% vs 6%], headache and breast pain, but “there was no significant difference among the groups in the incidence of abnormal liver-function tests”).
- Richardson AR, Maltz FN. Ulipristal acetate: review of the efficacy and safety of a newly approved agent for emergency contraception. Clin Ther. 2012;34:24–36. [PubMed: 22154199](Systematic review of safety and efficacy of emergency contraception using ulipristal or levonorgestrel reported no “clinically significant changes in biochemical parameters”).
- Plan B one-step OTC. Med Lett Drugs Ther. 2013;55(1419):52. [PubMed: 23797799](Concise review of efficacy and safety of single vs two dose levonorgestrel regimens for emergency contraception; mentions common adverse events of headache, abdominal pain and breast tenderness, but does not mention ALT elevations or hepatotoxicity).
- Turok DK, Jacobson JC, Dermish AI, Simonsen SE, Gurtcheff S, McFadden M, Murphy PA. Emergency contraception with a copper IUD or oral levonorgestrel: an observational study of 1-year pregnancy rates. Contraception. 2014;89:222–8. [PMC free article: PMC4076674] [PubMed: 24332433](Among 542 women requesting emergency contraception who were treated with oral levonorgestrel or implantation of a copper IUD, the one year pregnancy rates were 12% vs 7% and there were no reports of serious adverse events, ALT elevations or hepatotoxicity).
- Levy DP, Jager M, Kapp N, Abitbol JL. Ulipristal acetate for emergency contraception: postmarketing experience after use by more than 1 million women. Contraception. 2014;89:431–3. [PubMed: 24508124](A summary of spontaneous reports of adverse events after use of ulipristal for emergency contraception in more than one million women mentions side effects of nausea, headache, abdominal pain, and dizziness, but not ALT elevations or liver related adverse events).
- Cleland K, Raymond EG, Westley E, Trussell J. Emergency contraception review: evidence-based recommendations for clinicians. Clin Obstet Gynecol. 2014;57:741–50. [PMC free article: PMC4216625] [PubMed: 25254919](Review of emergency contraception mentions that the failure rates of ulipristal range from 0.9-2.1% and of levonorgestrel 0.6-3.1%, although both are less effective when given as an advance supply; in addition, they “have an excellent safety profile, and no deaths or serious complications have been causally linked” to their use).
- Donnez J, Hudecek R, Donnez O, Matule D, Arhendt HJ, Zatik J, Kasilovskiene Z, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103:519–27.e3. [PubMed: 25542821](Among 451 patients with symptomatic uterine fibroids treated with two 12-week courses of ulipristal [5 vs 10 mg daily], more than 80% had control of bleeding, and “laboratory results did not reveal any safety signals, and no patients discontinued treatment due to test abnormalities”).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury in the US collected between 2004 and 2012, no cases were attributed to emergency contraception regimens).
- Festin MP, Bahamondes L, Nguyen TM, Habib N, Thamkhantho M, Singh K, Gosavi A, et al. A prospective, open-label, single arm, multicentre study to evaluate efficacy, safety and acceptability of pericoital oral contraception using levonorgestrel 1.5 mg. Hum Reprod. 2016;31:530–40. [PMC free article: PMC4755445] [PubMed: 26830816](Among 330 women at risk of pregnancy given levonorgestrel tablets [1.5 mg] to use within 24 hours of intercourse, the pregnancy rate was 7.5 per 100 years of use and there were 3 serious adverse events [choledocholithiasis, anemia, and rupture of a corpus luteum cyst]; no mention of ALT elevations or hepatotoxicity).
- Fauser BC, Donnez J, Bouchard P, Barlow DH, Vázquez F, Arriagada P, Skouby SO, et al. Safety after extended repeated use of ulipristal acetate for uterine fibroids. PLoS One. 2017;12:e0173523. [PMC free article: PMC5340384] [PubMed: 28267814](Among 64 women with uterine fibroids treated with 1 to 8 three-month courses of ulipristal [10 mg daily], there were no changes in mean ALT or AST levels or in the “number of laboratory results falling outside of the normal range”).
- Rabe T, Saenger N, Ebert AD, Roemer T, Tinneberg HR, De Wilde RL, Wallwiener M. Selective progesterone receptor modulators for the medical treatment of uterine fibroids with a focus on ulipristal acetate. Biomed Res Int. 2018;2018:1374821. [PMC free article: PMC6261240] [PubMed: 30539001](Review of the efficacy and safety of ulipristal as therapy of uterine fibroids).
- Donnez J. Liver injury and ulipristal acetate: an overstated tragedy? Fertil Steril. 2018;110:593–5. [PubMed: 30196943](Brief summary of the results of liver test monitoring in the preregistration trials of ulipristal which showed no signal for potential hepatotoxicity).
- Ulipristal acetate (Esmya): restrictions on use. Drug Ther Bull. 2018;56:127. [PubMed: 30297449](Summary of a European Union wide review of ulipristal that was initiated after receipt of 8 reports of severe liver injury among an estimated 750,000 women treated with the agent for uterine fibroids, which resulted in recommendations to restrict use of ulipristal [to women with normal liver tests and who have refractory, moderate-to-severe, symptomatic fibroids] and require monitoring of liver tests before, during and after each course of therapy).
- Donnez J, Arriagada P, Marciniak M, Larrey D. Liver safety parameters ofulipristal acetate for the treatment of uterine fibroids: a comprehensive review of the clinical development program. Expert Opin Drug Saf. 2018;17:1225–32. [PubMed: 30460871](Detailed analysis of the changes in serum ALT, AST, Alk P and bilirubin levels in the preregistration trials of ulipristal during which there were no elevations in ALT above 3 times ULN among 422 women who received 5 mg daily and only 7 of 631 who received 10 mg daily, and no instance of clinically apparent liver injury at any dosage).
- Donnez J, Courtoy GE, Dolmans MM. Fibroid management in premenopausal women. Climacteric. 2019;22:27–33. [PubMed: 30601065](Review of clinical features and management of uterine fibroids in premenopausal women mentions that ulipristal “is safe, provides fast control of bleedings, and causes sustained fibroid volume reduction in the vast majority of cases”).
- Del Forno S, Degli Esposti E, Salucci P, Leonardi D, Iodice R, Arena A, Raimondo D, et al. Liver function, tolerability and satisfaction during treatment with ulipristal acetate in women with fibroids: a single center experience. Gynecol Endocrinol. 2020;36:445–7. [PubMed: 31646908](Among 162 women treated with at least one 3-month course of ulipristal [5 mg daily] for uterine fibroids, “no cases of increased serum AST or ALT levels were detected”).
- Meunier L, Meszaros M, Pageaux GP, Delay JM, Herrero A, Pinzani V, Dominique HB. Acute liver failure requiring transplantation caused by ulipristal acetate. Clin Res Hepatol Gastroenterol 2020: S2210-7401(20)30041-3. [PubMed: 32146092](58 year old woman with uterine fibroids developed fatigue 2 months after starting ulipristal followed by jaundice a week after stopping [bilirubin 25.5 mg/dL, ALT 1652 U/L, GGT 252 U/L, INR 2.5], with progressive hepatic failure leading to urgent liver transplantation; publication includes a table summarizing clinical features of 3 other cases of acute liver failure and liver transplantation in women arising 1-6 months after starting ulipristal for uterine fibroids).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Contraception for the older woman.[J Obstet Gynaecol (Lahore). 1985]Contraception for the older woman.Guillebaud J. J Obstet Gynaecol (Lahore). 1985 Jan; 5 Suppl 2:S70-7.
- Review Emergency contraception: a review.[Contraception. 1994]Review Emergency contraception: a review.Haspels AA. Contraception. 1994 Aug; 50(2):101-8.
- Review Contraception for women with diabetes: an update.[Baillieres Clin Obstet Gynaeco...]Review Contraception for women with diabetes: an update.Skouby SO, Mølsted-Pedersen L, Petersen KR. Baillieres Clin Obstet Gynaecol. 1991 Jun; 5(2):493-503.
- The effect of deliberate omission of Trinordiol or Microgynon on the hypothalamo-pituitary-ovarian axis.[Contraception. 1986]The effect of deliberate omission of Trinordiol or Microgynon on the hypothalamo-pituitary-ovarian axis.Smith SK, Kirkman RJ, Arce BB, McNeilly AS, Loudon NB, Baird DT. Contraception. 1986 Nov; 34(5):513-22.
- Emergency contraception: is it time to change method?[BMJ. 1999]Emergency contraception: is it time to change method?Webb A. BMJ. 1999 Feb 6; 318(7180):342-3.
- Emergency Contraceptive Agents - LiverToxEmergency Contraceptive Agents - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...