NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Eribulin mesylate is an inhibitor of microtubule function and is used as an antineoplastic agent for refractory, metastatic breast cancer and liposarcoma. Despite its cytotoxic activity against cancer cells, eribulin has rarely been implicated in causing clinically apparent acute liver injury.
Background
Eribulin (er' i bue' lin) is a synthetic macrocyclic analogue of halichondrin B, a naturally occurring inhibitor of mitotic division found in a species of marine sponges (Halichondria okadai). Eribulin binds to the growing ends of microtubules preventing cell division (mitotic arrest) which leads to tubulin sequestration in aggregates and apoptotic cell death. Eribulin was approved for use in cancer chemotherapy in 2010 and current indications are for metastatic breast cancer after failure of chemotherapy with an anthracycline and taxane. It is also approved for use in metastatic, refractory liposarcoma. Eribulin is given intravenously in doses of 1.4 mg/m2, typically on days 1 and 8 of 21 day cycles. Eribulin is available in single use vials of 1 mg per 2 mL under the trade name Halaven. Side effects are common and include nausea, vomiting, fatigue, headache, dizziness, peripheral neuropathy, hoarseness, ataxia, dysphagia, urinary retention, constipation, diarrhea, bone marrow suppression, alopecia and phlebitis at the infusion site. Rare, but potentially severe adverse events include severe neutropenia, peripheral neuropathy, prolongation of the QTc interval and embryo-fetal toxicity.
Hepatotoxicity
Eribulin is a cytotoxic chemotherapeutic agent and serum aminotransferase and alkaline phosphatase elevations are common during cyclic therapy of breast cancer and liposarcoma. The reported rates of ALT elevations have ranged from 8% to 83% with values above 5 times the upper limit or normal (ULN) in 2% to 5%. Isolated mention of “toxic hepatitis” have been made in reports of clinical trials of eribulin but no details of the onset, clinical features and course have been published and the role of eribulin in these outcomes is uncertain. Nevertheless, despite high rates of serum enzyme elevations during treatment, cases of clinically apparent liver injury have not been reported in detail and must be rare.
Likelihood score: E* (unproven but suspected cause of liver injury).
Mechanism of Injury
Eribulin causes mitotic arrest in rapidly dividing cells and likely causes similar changes in some hepatocytes, perhaps accounting for the frequent occurrence of mild serum aminotransferase elevations during therapy. Eribulin undergoes minimal hepatic metabolism and is mostly excreted unchanged. It also has little or no effect on drug metabolism and minimal drug-drug interactions.
Outcome and Management
Serum enzyme elevations are frequent during cancer chemotherapy with eribulin, but are rarely dose limiting. Serum aminotransferase elevations above 5 times ULN should lead to dose interuption or modification. Serum ALT or AST elevations above ten times ULN or any elevations accompanied by symptoms of liver disease or jaundice should lead to discontinuation of eribulin therapy, but should also trigger a search for other causes.
Drug Class: Antineoplastic Agents, Miscellaneous, Microtubule Inhibitors
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Eribulin – Halaven®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
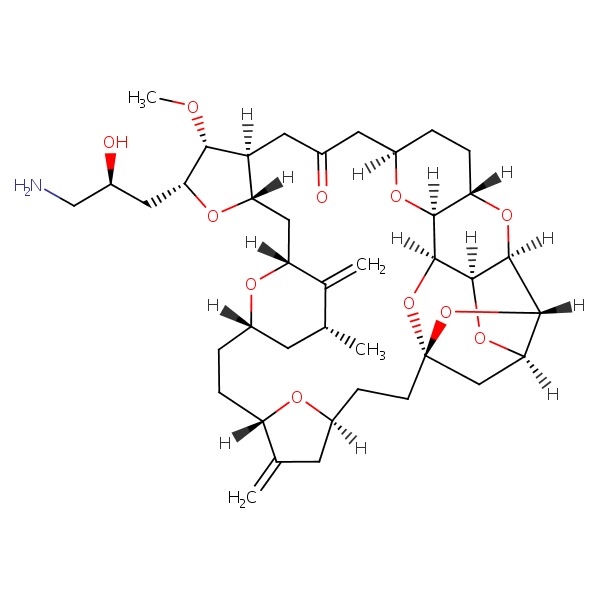
| Eribulin | 253128-41-5 | C40-H59-N-O11 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 20 February 2018
- Zimmerman HJ. The vinca alkaloids. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 692-4.(Expert review of hepatotoxicity published in 1999 before the availability of eribulin, which is not discussed).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 549-68.(Review of hepatotoxicity of published in 2013; eribulin is not discussed).
- Chabner BA, Bertino J, Clearly J, Ortiz T, Lane A, Supko JG, Ryan DP. Cytotoxic agents. Chemotherapy of neoplastic diseases. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1677-1730.(Textbook of pharmacology and therapeutics).
- Cortes J, Vahdat L, Blum JL, Twelves C, Campone M, Roché H, Bachelot T, et al. Phase II study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine. J Clin Oncol 2010; 28: 3922-8. [PubMed: 20679609](Among 291 patients with refractory metastatic breast cancer treated with eribulin, the objective response rate was 9% and toxicity was “manageable”, side effects included fatigue [65%], neutropenia [60%], alopecia [60%], neuropathy [33%]; no mention of ALT elevations or hepatotoxicity).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury and 2 to antineoplastic agents [melphalan and gemtuzumab], but none to eribulin).
- Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, et al.; EMBRACE (Eisai Metastatic Breast Cancer Study Assessing Physician's Choice Versus E7389) investigators. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomized study. Lancet 2011; 377 (9769): 914-23. [PubMed: 21376385](Among 762 women with metastatic breast cancer treated with eribulin or standard therapy, overall survival was improved with eribulin [13.1 vs 10.6 months] and adverse events were similar, although neutropenia and neuropathy were more frequent with eribulin; no mention of ALT elevations or hepatotoxicity).
- Schöffski P, Ray-Coquard IL, Cioffi A, Bui NB, Bauer S, Hartmann JT, Krarup-Hansen A, et al.; European Organisation for Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group (STBSG). Activity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histological subtypes. Lancet Oncol 2011; 12: 1045-52. [PubMed: 21937277](Among 128 patients with soft tissue sarcomas treated with eribulin, common severe side effects included neutropenia [52%], anemia [7%] and ALT elevations [57%], which were above 5 times ULN in 6 patients [5%], but none developed clinically apparent liver injury).
- Eribulin mesylate (Halaven) for breast cancer. Med Lett Drugs Ther 2011; 53 (1362): 30-1. [PubMed: 21502935](Concise review of the mechanism of action, efficacy, safety and costs of eribulin shortly after its approval for use in the US, mentions that adverse events include neutropenia, anemia, fatigue, neuropathy, nausea and constipation; no mention of ALT elevations or hepatotoxicity).
- Aogi K, Iwata H, Masuda N, Mukai H, Yoshida M, Rai Y, Taguchi K, et al. A phase II study of eribulin in Japanese patients with heavily pretreated metastatic breast cancer. Ann Oncol 2012; 23: 1441-8. [PubMed: 21989327](Among 80 Japanese women with refractory metastatic breast cancer treated with eribulin, the objective response rate was 21% and common side effects were neutropenia [98%], alopecia [58%], fatigue [46%], neuropathy [23%], ALT elevations [33%: 4% were greater than 5 times ULN] and Alk P elevations [21%]).
- de Bono JS, Molife LR, Sonpavde G, Maroto JP, Calvo E, Cartwright TH, Loesch DM, et al. Phase II study of eribulin mesylate (E7389) in patients with metastatic castration-resistant prostate cancer stratified by prior taxane therapy. Ann Oncol 2012; 23: 1241-9. [PubMed: 21903605](Among 108 men with refractory metastatic prostate cancer treated with eribulin, common side effects were neutropenia, fatigue and peripheral neuropathy; no mention of ALT elevations or hepatotoxicity).
- Wilks S, Puhalla S, O'Shaughnessy J, Schwartzberg L, Berrak E, Song J, Cox D, Vahdat L. Phase 2, multicenter, single-arm study of eribulin mesylate with trastuzumab as first-line therapy for locally recurrent or metastatic HER2-positive breast cancer. Clin Breast Cancer 2014; 14: 405-12. [PubMed: 25024001](Among 52 patients with metastatic breast cancer treated with the combination of eribulin and trastuzumab, the objective response rate was 71% and ALT elevations above 5 times ULN occurred in only one patient [2%]).
- McIntyre K, O'Shaughnessy J, Schwartzberg L, Glück S, Berrak E, Song JX, Cox D, et al. Phase 2 study of eribulin mesylate as first-line therapy for locally recurrent or metastatic human epidermal growth factor receptor 2-negative breast cancer. Breast Cancer Res Treat 2014; 146: 321-8. [PMC free article: PMC4085472] [PubMed: 24699910](Among 56 women with recurrent or metastatic breast cancer treated with eribulin, the objective response rate was 29% and side effects were common, including fatigue, neutropenia and neuropathy; no mention of ALT elevations or hepatotoxicity).
- Kaufman PA, Awada A, Twelves C, Yelle L, Perez EA, Velikova G, Olivo MS, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 2015; 33: 594-601. [PMC free article: PMC4463422] [PubMed: 25605862](Among 1102 women with previously treated metastatic breast cancer treated with eribulin or capecitabine, the objective response rate was similar in the 2 groups, but the duration of response was longer with capecitabine; ALT elevations occurred in 8.5% vs 4.2% and were above 5 times ULN in 3.3% vs 0.5%; one patient treated with eribulin died of “toxic hepatitis”).
- Goodin S, Barbour S, Song J, Berrak E, Cox D. Safety and tolerability of eribulin mesylate in patients with pretreated metastatic breast cancer. Am J Health Syst Pharm 2015; 72: 2150-6. [PubMed: 26637514](Pooled analysis of results from 3 phase II and 1 phase III trial of eribulin in women with previously treated, metastatic breast cancer found that ALT elevations arose in 5% of patients, but none [that were attributable to eribulin] were above 5 times ULN).
- Garrone O, Montemurro F, Saggia C, La Verde N, Vandone AM, Airoldi M, De Conciliis E, et al. Eribulin in pretreated metastatic breast cancer patients: results of the TROTTER trial-a multicenter retrospective study of eribulin in real life. Springerplus 2016; 5: 59. [PMC free article: PMC4720621] [PubMed: 26835238](Among 113 Italian “real life” women with metastatic breast cancer treated with eribulin, the objective response rate was 24% and toxicity was “manageable”; liver test abnormalities occurred in 18 [16%] and were above 5 times ULN in 2 [2%]).
- Takashima T, Tokunaga S, Tei S, Nishimura S, Kawajiri H, Kashiwagi S, Yamagata S, et al. A phase II, multicenter, single-arm trial of eribulin as first-line chemotherapy for HER2-negative locally advanced or metastatic breast cancer. Springerplus 2016; 5: 164. [PMC free article: PMC4766136] [PubMed: 27026861](Among 35 Japanese women with metastatic breast cancer treated with eribulin as a first line therapy, the objective response rate was 54% and adverse events were “acceptable”; ALT and AST elevations occurred in 29 patients [83%], but were above 5 times ULN in only one [3%]).
- Kobayashi T, Tomomatsu J, Fukada I, Shibayama T, Teruya N, Ito Y, Iwase T, et al. Eribulin-induced liver dysfunction as a prognostic indicator of survival of metastatic breast cancer patients: a retrospective study. BMC Cancer 2016; 16: 404. 27389013. [PMC free article: PMC4936231] [PubMed: 27389013](Among 151 women with metastatic breast cancer treated with eribulin between 2011 and 2013, 42 [28%] developed ALT or AST elevations above 3 times ULN after a median time of 21 days, these elevations leading to dose interruption or modification in 10 and permanent discontinuation in 1 patient; nevertheless, women with such enzyme elevations had a better overall and progression free survival).
- Bunchorntavakul C, Reddy KR. Drug hepatotoxicity: newer qgents. Clin Liver Dis 2017; 21: 115-34. 27842767. [PubMed: 27842767](Review of the hepatotoxicity of recently approved medications including tryosine kinase receptor inhibitors and monoclonal antibodies does not specifically discuss eribulin).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Review on the clinical use of eribulin mesylate for the treatment of breast cancer.[Expert Opin Pharmacother. 2016]Review Review on the clinical use of eribulin mesylate for the treatment of breast cancer.Aseyev O, Ribeiro JM, Cardoso F. Expert Opin Pharmacother. 2016; 17(4):589-600. Epub 2016 Feb 17.
- Review Eribulin mesylate: a novel halichondrin B analogue for the treatment of metastatic breast cancer.[Am J Health Syst Pharm. 2012]Review Eribulin mesylate: a novel halichondrin B analogue for the treatment of metastatic breast cancer.McBride A, Butler SK. Am J Health Syst Pharm. 2012 May 1; 69(9):745-55.
- Review Eribulin mesylate (E7389): review of efficacy and tolerability in breast, pancreatic, head and neck, and non-small cell lung cancer.[Clin Ther. 2012]Review Eribulin mesylate (E7389): review of efficacy and tolerability in breast, pancreatic, head and neck, and non-small cell lung cancer.Scarpace SL. Clin Ther. 2012 Jul; 34(7):1467-73. Epub 2012 Jun 25.
- Review Eribulin mesylate.[Clin Cancer Res. 2011]Review Eribulin mesylate.Jain S, Vahdat LT. Clin Cancer Res. 2011 Nov 1; 17(21):6615-22. Epub 2011 Aug 22.
- Health-related quality of life in patients receiving first-line eribulin mesylate with or without trastuzumab for locally recurrent or metastatic breast cancer.[BMC Cancer. 2019]Health-related quality of life in patients receiving first-line eribulin mesylate with or without trastuzumab for locally recurrent or metastatic breast cancer.Schwartzberg L, McIntyre K, Wilks S, Puhalla S, O'Shaughnessy J, Berrak E, He Y, Vahdat L. BMC Cancer. 2019 Jun 13; 19(1):578. Epub 2019 Jun 13.
- Eribulin - LiverToxEribulin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...