NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Gabapentin is a unique anticonvulsant that is used as adjunctive therapy in management of epilepsy and for neuropathic pain syndromes. Therapy with gabapentin is not associated with serum aminotransferase elevations, but several cases of clinically apparent liver injury from gabapentin have been reported.
Background
Gabapentin (gab" a pen' tin) is a structural analogue of gamma-aminobutyric acid (GABA), but demonstrates little or no interaction with GABA receptors and does not appear to alter GABA uptake, synthesis or metabolism. While initially believed to act on the GABA-ergic neurotransmitter system, the actual mechanism of action of gabapentin as an anticonvulsant and agent for neuropathy is unknown. Gabapentin was approved for use in the United States in 1993 and is a widely used medication with more than 18 million prescriptions filled yearly. Current indications include add-on therapy for partial seizures which do not have adequate control with other anticonvulsants, and to reduce neuropathic pain from diabetic and postherpetic neuropathy. Gabapentin is available as capsules or tablets of 100, 300, 400, 600 and 800 mg and in oral solution for pediatric use generically and under the brand names Neurontin and Gabarone. The recommended initial dose for adults is 300 mg three times daily increasing as needed to a maximum dose of 1800 mg daily. The most common side effects of gabapentin are dose related and include dizziness, somnolence, tremor, ataxia, blurred vision, anxiety, and gastrointestinal upset or nausea. Rare, but potentially severe adverse events include hypersensitivity reactions such as angioneurotic edema, drug rash with eosinophilia with systemic manifestations (DRESS syndrome) and Stevens-Johnson syndrome.
Hepatotoxicity
Limited data are available on the hepatotoxicity of gabapentin. In clinical trials in diabetic neuropathy and epilepsy, therapy with gabapentin was not associated with an increased frequency of serum aminotransferase elevations or liver toxicity. Rare individual case reports of liver injury from gabapentin have been published, although the causal relationship of gabapentin with the liver injury was not always clear. The latency to onset in these reports was 1 to 8 weeks and associated with cholestatic pattern of enzyme elevations. Fever and rash have been described but not autoantibody formation. Reported cases have been mild to moderate in severity and self-limited in course. In view of the wide-scale use of gabapentin, liver injury with symptoms or jaundice is clearly quite rare.
Likelihood score: C (probable cause of clinically apparent liver injury).
Mechanism of Injury
The apparent absence or low rate of significant hepatotoxicity from gabapentin may be due to its minimal hepatic metabolism and rapid urinary excretion.
Outcome and Management
The case reports of hepatic injury due to gabapentin were followed by complete recovery without evidence of residual or chronic injury. No cases of acute liver failure or chronic liver injury due to gabapentin have been described. There is no information about cross reactivity with other compounds having similar structure (pregabalin). In general, gabapentin is well tolerated in patients with hypersensitivity reactions to other anticonvulsants.
Drug Class: Anticonvulsants
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Gabapentin – Generic, Neurontin®
DRUG CLASS
Anticonvulsants
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
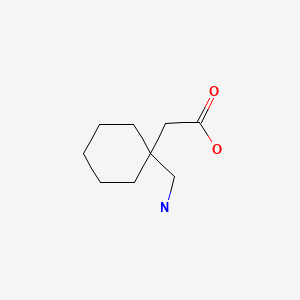
| Gabapentin | 60142-96-3 | C9-H17-N-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 30 July 2020
- Zimmerman HJ. Anticonvulsants. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 498-516.(Expert review of anticonvulsants and liver injury published in 1999; gabapentin is not discussed).
- Pirmohamed M, Leeder SJ. Anticonvulsant agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp 423-42.(Review of anticonvulsant induced liver injury; gabapentin is not discussed).
- Smith MD, Metcalf CS, Wilcox KS. Pharmacology of the epilepsies. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 303-26.(Textbook of pharmacology and therapeutics).
- Gabapentin Chadwick D. Lancet. 1994;343:89–91. [PubMed: 7903783](Review of the clinical uses of gabapentin largely as add-on to other anticonvulsants. Most common side effects were somnolence, dizziness, ataxia, fatigue, nystagmus, headache, tremor, diplopia, nausea and rhinitis. “No serious idiosyncratic reactions have been identified with gabapentin and, in particular, there is no evidence of hypersensitivity reactions…”).
- US Gabapentin Study Group. The long-term safety and efficacy of gabapentin (Neurontin) as add-on therapy in drug-resistant partial epilepsy. Epilepsy Res. 1994;18:67–73. [PubMed: 8088258](Systematic review; ALT elevations occur in 4% of children on phenytoin, 6% on valproate, 1% on carbamazepine, but none reported on tiagabine or gabapentin).
- Hamer HM, Morris HH. Hypersensitivity syndrome to antiepileptic drugs: a review including new anticonvulsants. Cleve Clin J Med. 1999;66:239–45. [PubMed: 10199060](Clinical review of anticonvulsant hypersensitivity syndrome, which occurs in 1 to 5 per 10,000 users of aromatic anticonvulsants with higher risk in African Americans and affected siblings; liver involvement common, but most cases are anicteric; other manifestations include facial edema, lymphadenopathy, bone marrow aplasia, pseudolymphoma, thyroiditis, interstitial nephritis; not linked to gabapentin).
- Hamer HM, Morris HH. Successful treatment with gabapentin in the presence of hypersensitivity syndrome to phenytoin and carbamazepine: a report of three cases. Seizure. 1999;8:190–2. [PubMed: 10356381](3 patients developed rash, fever, lymphadenopathy and eosinophilia 4-6 weeks after starting either phenytoin or carbamazepine [bilirubin 0.5-1.8 mg/dL, ALT 866-1402 U/L, Alk P 69-364 U/L], resolving after stopping and not recurring during gabapentin therapy).
- Wong ICK, Lhatto SD. Adverse reactions to new anticonvulsant drugs. Drug Safety. 2000;23:35–56. [PubMed: 10915031](Review of side effects of new anticonvulsants: side effects of gabapentin are largely CNS related and include somnolence, dizziness, diplopia, ataxia, tremor and nausea, but no mention of liver toxicity and overdose was associated with lethargy only).
- Ragucci MV, Cohen JM. Gabapentin-induced hypersensitivity syndrome. Clin Neuropharmacol. 2001;24:103–5. [PubMed: 11307046](72 year old man developed confusion and fever 8 days after starting gabapentin; was then given levofloxacin and developed rash liver abnormalities 2 days later [bilirubin normal, GGT 296 U/L, Alk P 218 U/L], resolving 15 days after stopping gabapentin; case did not fulfill criteria for hypersensitivity syndrome and was complicated by use of levofloxacin).
- Lasso-de-la-Vega MC, Zapater P, Such J, Pérez-Mateo M, Horga JF. Gabapentin-associated hepatotoxicity. Am J Gastroenterol. 2001;96:3460–2. [PubMed: 11774985](60 year old man developed skin rash and eosinophilia 1 month after starting gabapentin and 1 day after starting ciprofloxacin; 1 week later was found to be jaundiced [bilirubin not given, ALT ~320 U/L, GGT ~1000 U/L], gabapentin was decreased in dose and stopped a week later and laboratory tests improved concurrently: ciprofloxacin might also have been responsible).
- Hauben M. Re: Lasso-de-la-Vega et al. Gabapentin as a probable cause of hepatotoxicity and eosinophilia. Am J Gastroenterol. 2002;97:2156–7. [PubMed: 12190207](Letter from industry sponsor suggesting that hypersensitivity reaction in patient described by Lasso-de-la-Vega [2001] was more likely due to ciprofloxacin than gabapentin).
- Bureau C, Poirson H, Péron JM, Vinel JP. Gastroenterol Clin Biol. 2003;27:1169–70. [Gabapentine-induced acute hepatitis] French. [PubMed: 14770125](Patient developed jaundice, fever and pain 2 months after starting gabapentin, had inflamed gallbladder and underwent cholecystectomy, but bilirubin continued to rise [bilirubin either 2.7 or 27 mg/dL, ALT 8 times ULN, Alk P 12 times ULN]; gabapentin was stopped, ultimate complete recovery in ~1 month; unclear whether gabapentin or gallstones were the cause).
- Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, Mason A, et al. Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation. Health Technol Assess. 2005;9:1–157. iii-iv. [PubMed: 15842952](Extensive systematic review of anticonvulsant medications including assessment of serious, rare and long term adverse events; severe side effects are rare with gabapentin; no mention of hepatotoxicity).
- LaRoche SM. A new look at the second-generation antiepileptic drugs: a decade of experience. Neurologist. 2007;13:133–9. [PubMed: 17495757](Review of second generation anticonvulsants approved since 1994 including felbamate, gabapentin, lamotrigine, topiramate, tiagabine, levetiracetam, oxcarbazepine, zonisamide and pregabalin; serious side effects are rare and no mention of liver toxicity from gabapentin).
- Himmerich H, Nickel T, Dalal MA, Müller MB. Psychiatr Prax. 2007;34:93–4. [Gabapentin treatment in a female patient with panic disorder and adverse effects under carbamazepine during benzodiazepine withdrawal] German. [PubMed: 17124639](70 year old woman with panic disorder and benzodiazepine dependence who developed liver test abnormalities on carbamazepine [ALT 103 U/L, GGT 309 U/L] tolerated long term therapy with gabapentin, which allowed withdrawal of benzodiazepines without recurrence of ALT elevations).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, valproate accounted for 6, lamotrigine 5, phenytoin 5, gabapentin and topiramate 1 each but details not provided).
- Björnsson E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scand. 2008;118:281–90. [PubMed: 18341684](Review of all anticonvulsant induced liver injury; neither gabapentin nor pregabalin are discussed).
- Drugs for epilepsy. Treat Guidel Med Lett. 2013;11:9–18. Erratum in Treat Guidel Med Lett 2013; 11: 112. [PubMed: 23348233](Concise review of drugs of choice for epilepsy; gabapentin is FDA approved as adjunctive therapy of partial seizures and adverse effects include somnolence, dizziness, ataxia, fatigue, and blurred vision; no mention of hepatotoxicity or ALT elevations).
- Fuzier R, Serres I, Guitton E, Lapeyre-Mestre M, Montastruc JL., French Network of Pharmacovigilance Centres. Adverse drug reactions to gabapentin and pregabalin: a review of the French pharmacovigilance database. Drug Saf. 2013;36:55–62. [PubMed: 23315296](Among 725 spontaneous adverse event reports related to gabapentin made to the French Pharmacovigilance System between 1995 and 2009, liver ranked second to neuropsychiatric reactions in frequency [n=90, 12%], 37 of which were “hepatitis”, half of which were serious, 8 were “probable” or “likely” and one fatal, but no specific details given).
- Gabapentin and pregabalin: hepatic and haematological toxicity. Prescrire Int. 2014;23(154):267. [PubMed: 25954794](Review of spontaneous reports of adverse events attributed to gabapentin from a French registry [Fuzier 2013] identified 90 cases of liver damage, gabapentin being the only suspect drug in 10 cases of "hepatitis", one of which was fatal).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 40 cases [4.5%] were attributed to anticonvulsants, including 3 to gabapentin, the latency to onset being 3-6 weeks, with a mixed or cholestatic pattern of injury and a moderate, self-limited course).
- Zaccara G, Giovannelli F, Giorgi FS, Franco V, Gasparini S, Benedetto U. Tolerability of new antiepileptic drugs: a network meta-analysis. Eur J Clin Pharmacol. 2017;73:811–7. [PubMed: 28378057](Metanalysis of the comparative tolerability of 18 new anticonvulsant agents including gabapentin, found lowest rates of withdrawal for adverse events with levetiracetam, brivaracetam, and gabapentin with highest rates with eslicarbazepine, oxcarbazepine, lacosamide and topiramate).
- Drugs for epilepsy. Med Lett Drugs Ther. 2017;59(1526):121–30. [PubMed: 28746301](Concise review of the drugs available for therapy of epilepsy lists gabapentin as a second line, alternative therapy of partial onset seizures and mentions common side effects of somnolence, dizziness, ataxia, fatigue, blurred vision and confusion, but does not mention ALT elevations or hepatotoxicity).
- Vidaurre J, Gedela S, Yarosz S. Antiepileptic drugs and liver disease. Pediatr Neurol. 2017;77:23–36. [PubMed: 29097018](Summary of the hepatotoxicity of major anticonvulsant medications including gabapentin which has no hepatic metabolism, so specific recommendation for dose modification because of liver disease, a minimal potential for drug interactions and low association with hepatotoxicity).
- Nonopioid drugs for pain. Med Lett Drugs Ther. 2018;60(1540):25–32. [PubMed: 29422479](Concise review of the mechanism of action, clinical efficacy, common side effects and costs of nonopioid drugs that are used for pain, mentions that gabapentin is approved for use in post-herpetic and diabetic neuropathy; no mention of hepatic adverse events).
- Borrelli EP, Lee EY, Descoteaux AM, Kogut SJ, Caffrey AR. Stevens-Johnson syndrome and toxic epidermal necrolysis with antiepileptic drugs: An analysis of the US Food and Drug Administration Adverse Event Reporting System. Epilepsia. 2018;59:2318–24. [PMC free article: PMC6420776] [PubMed: 30395352](Review of adverse event reports to the FDA between 2014 and 2018 identified ~2.9 million reports, 1034 for SJS/TEN, the most common class of drugs being anticonvulsants with 17 of 34 having at least one report, those most frequently linked being lamotrigine [n=106], carbamazepine [22], levetiracetam [14], phenytoin [14], valproate [9], clonazepam [8], zonisamide [7], gabapentin [4] and pregabalin [4]; no mention of accompanying liver injury or whether attribution was as a single agent or one of several).
- Cano-Paniagua A, Amariles P, Angulo N, Restrepo-Garay M. Epidemiology of drug-induced liver injury in a University Hospital from Colombia: Updated RUCAM being used for prospective causality assessment. Ann Hepatol. 2019;18:501–7. [PubMed: 31053545](Among 286 patients with liver test abnormalities seen in a single hospital in Colombia over a 1 year period, 17 were diagnosed with drug induced liver injury, the most common cause being antituberculosis therapy [n=6] followed by anticonvulsants [n=3, 1 each due to phenytoin, gabapentin and valproate]).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Gabapentin use in neuropathic pain syndromes.[Acta Neurol Scand. 2000]Review Gabapentin use in neuropathic pain syndromes.Nicholson B. Acta Neurol Scand. 2000 Jun; 101(6):359-71.
- Review Clorazepate.[LiverTox: Clinical and Researc...]Review Clorazepate.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Update on gabapentin therapy of neuropathic pain.[Consult Pharm. 2003]Update on gabapentin therapy of neuropathic pain.Guay DR. Consult Pharm. 2003 Feb; 18(2):158-70, 173-8.
- Gabapentin is a first line drug for the treatment of neuropathic pain in spinal cord injury.[Spine (Phila Pa 1976). 2004]Gabapentin is a first line drug for the treatment of neuropathic pain in spinal cord injury.Levendoglu F, Ogün CO, Ozerbil O, Ogün TC, Ugurlu H. Spine (Phila Pa 1976). 2004 Apr 1; 29(7):743-51.
- Review Clonazepam.[LiverTox: Clinical and Researc...]Review Clonazepam.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Gabapentin - LiverToxGabapentin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...