NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Gemifloxacin is a fourth generation, oral fluoroquinolone antibiotic used in the therapy of mild-to-moderate respiratory tract infections caused by susceptible organisms. Gemifloxacin has been linked to rare instances of acute liver injury.
Background
Gemifloxacin is an oral, fourth generation fluoroquinolone that is used to treat mild-to-moderate respiratory tract infections. Like other fluoroquinolones, gemifloxacin is active against a wide range of aerobic gram-positive and gram-negative organisms and is believed to act by inhibition of bacterial DNA gyrase and topoisomerase IV that are required for synthesis of bacterial mRNAs (transcription) and DNA replication. In contrast, DNA gyrases are not present in human [and other eukarotic] cells and the equivalent topoisomerases are not sensitive to fluoroquinolone inhibition. Gemifloxacin was approved for use in the United States in 2003 and has not been as commonly used as other fluoroquinolones such as ciprofloxacin and levofloxacin. Current indications are limited to acute exacerbations of chronic bronchitis and community acquired pneumonia. Gemifloxacin is available under the commercial name Factive in 320 mg tablets. The recommended dose is 320 mg once daily for 5 to 7 days. Common side effects include diarrhea, nausea, abdominal pain, headaches, skin rash and allergic reactions. The less common but more severe side effects of the fluoroquinolones include prolongation of the QT interval, seizures, hallucinations, tendon rupture, severe hypersensitivity reactions, Stevens Johnson syndrome, angioedema and photosensitivity.
Hepatotoxicity
Gemifloxacin (jem" i flox' a sin), like other fluoroquinolones, is associated with a low rate (1% to 7%) of serum enzyme elevations during therapy, although this rate may be slightly higher than occurs with placebo or comparative agents. These abnormalities are generally mild, asymptomatic and transient, resolving even with continuation of therapy. Too few cases of hepatotoxicity due to gemifloxacin have been reported to provide a reliable description of its clinical features and course. However, the liver injury due to the fluoroquinolones appears to be a class effect, and gemifloxacin injury appears to share these characteristics. It is a far less common cause of liver injury than ciprofloxacin, levofloxacin or moxifloxacin, but cases have been reported. In general, fluoroquinone hepatotoxicity is marked by a short time to onset (a few days to 3 weeks); often presenting abruptly with marked nausea, fatigue, abdominal pain and jaundice. The pattern of serum enzyme elevations can be either hepatocellular or cholestatic, and cases with the shorter times to onset are usually more hepatocellular with markedly elevated ALT levels, and occasionally, with rapid worsening of prothrombin time and signs of hepatic failure. The onset of illness may occur a few days after the medication is stopped. Many (but not all) cases have had allergic manifestations with fever, rash and eosinophilia. Autoantibodies are usually not present.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of fluoroquinolone hepatotoxicity is suspected to be hypersensitivity. Rechallenge leads to recurrence and should be avoided.
Outcome and Management
While few cases of clinically apparent liver injury due to gemifloxacin have been reported, such injury does occur with other fluoroquinolones, even with brief periods of treatment (2 to 5 days). The severity of injury ranges from mild, asymptomatic and transient serum enzyme elevations to a mild, prolonged cholestatic hepatitis, to self-limited acute hepatocellular jaundice and even to acute liver failure. Cross reactivity of the hepatic injury between different fluoroquinolones has been demonstrated in a small number of cases, but should be assumed based upon the similarity of clinical patterns of injury and latency.
Drug Class: Antiinfective Agents
Other Drugs in the Subclass, Fluoroquinolones: Ciprofloxacin, Delafloxacin, Levofloxacin, Moxifloxacin, Norfloxacin, Ofloxacin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Gemifloxacin – Factive®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
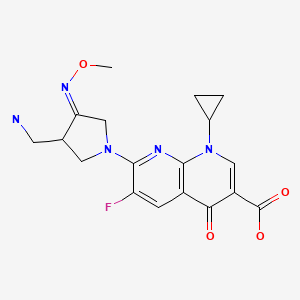
| Gemifloxacin | 175463-14-6 | C18-H20-F-N5-O4 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 10 March 2020
- Zimmerman HJ. Quinolones. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 603.(Expert review of hepatotoxicity published in 1999, before the availability of gemifloxacin, mentions that cinoxacin, nalidixic acid, ciprofloxacin, norfloxacin, enoxacin, and ofloxacin are associated with minor serum enzyme elevations during therapy and with rare instances of clinically apparent liver injury; gemifloxacin is not discussed).
- Moseley RH. Fluoroquinolones. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 468.(Review of hepatotoxicity of fluoroquinolones mentions that, although rare, hepatocellular and cholestatic forms of fluoroquinolone associated clinically apparent liver injury have been reported including cases of ductopenia, acute liver failure and death; gemifloxacin is not specifically discussed).
- MacDougall C. The quinolones. Sulfonamides, trimethoprim-sulfamethoxazole, quinolones, and agents for urinary tract infections. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1015-8.(Textbook of pharmacology and therapeutics).
- Bertino J Jr, Fish D. The safety profile of the fluoroquinolones. Clin Ther. 2000;22:798–817. [PubMed: 10945507](Systematic review of literature on safety of fluoroquinolones; most common side effects are gastrointestinal complaints [1-8%]; rare side effects include anaphylaxis, prolongation of QTc interval, tendon rupture and CNS toxicity; trovafloxacin has been linked to several cases of acute liver failure; no discussion of gemifloxacin).
- File TM Jr, Schlemmer B, Garau J, Cupo M, Young C. 049 Clinical Study Group. Efficacy and safety of gemifloxacin in the treatment of community-acquired pneumonia: a randomized, double-blind comparison with trovafloxacin. J Antimicrob Chemother. 2001;48:67–74. [PubMed: 11474633](Controlled trial comparing oral gemifloxacin vs trovafloxacin in 571 patients with community acquired pneumonia found similar rates of response and side effects; ALT or AST elevations occurred in 2.1% of trovafloxacin treated [peak ALT 184 U/L] vs 0.3% of gemifloxacin treated [peak ALT 139 U/L], all of which resolved once therapy was stopped).
- Lode H, File TM Jr, Mandell L, Ball P, Pypstra R, Thomas M. 185 Gemifloxacin Study Group. Oral gemifloxacin versus sequential therapy with intravenous ceftriaxone/oral cefuroxime with or without a macrolide in the treatment of patients hospitalized with community-acquired pneumonia: a randomized, open-label, multicenter study of clinical efficacy and tolerability. Clin Ther. 2002;24:1915–36. [PubMed: 12501883](Controlled trial of oral gemifloxacin vs iv followed by oral ceftriaxone for 7-14 days in 341 patients with community acquired pneumonia; liver test elevations occurred in 7 [4.1%] gemifloxacin vs 12 [7%] ceftriaxone treated subjects, but no mention of clinically apparent liver injury).
- Ball P, Mandell L, Patou G, Dankner W, Tillotson G. A new respiratory fluoroquinolone, oral gemifloxacin: a safety profile in context. Int J Antimicrob Agents. 2004;23:421–9. [PubMed: 15120718](Review of safety data from 6775 patients treated with gemifloxacin in clinical trials; frequency of adverse events was similar to that in comparator arms using other antibiotics, ALT elevations in 1.5% with gemifloxacin and 0.9% with comparators and "no significant drug-related liver reactions were observed").
- Bhavnani SM, Andes DR. Gemifloxacin for the treatment of respiratory tract infections: in vitro susceptibility, pharmacokinetics and pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy. 2005;25:717–40. [PubMed: 15899734](Review of pharmacokinetics, efficacy and safety of gemifloxacin; most common side effects are diarrhea, rash, nausea and headache; increased AST values occurred in 0.3% of treated patients in one study).
- File TM Jr, Mandell LA, Tillotson G, Kostov K, Georgiev O. Gemifloxacin once daily for 5 days versus 7 days for the treatment of community-acquired pneumonia: a randomized, multicentre, double-blind study. J Antimicrob Chemother. 2007;60:112–20. [PubMed: 17537866](Controlled trial comparing 5 and 7 days of oral gemifloxacin in 469 patients with community acquired pneumonia found similar rates of response and tolerance; ALT elevations occurred in 6.1% of patients, but no patient developed clinically apparent liver injury).
- Lode HM, Schmidt-Ionas M, Stahlmann R. Gemifloxacin for community-acquired pneumonia. Expert Opin Investig Drugs. 2008;17:779–86. [PubMed: 18447602](Systematic review of safety and efficacy of gemifloxacin; rates of common side effects with gemifloxacin are similar to those of comparator fluoroquinolones; ALT elevations occur in 4.7-7.4% with 5 to 7 day course of oral therapy).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury but only one to a fluoroquinolone, ciprofloxacin).
- Orman ES, Conjeevaram HS, Vuppalanchi R, Freston JW, Rochon J, Kleiner DE, Hayashi PH., DILIN Research Group. Clinical and histopathologic features of fluoroquinolone-induced liver injury. Clin Gastroenterol Hepatol. 2011;9:517–523.e3. [PMC free article: PMC3718017] [PubMed: 21356330](Among 679 cases of drug induced liver injury presenting between 2004 and 2010 at 8 US medical centers, 12 [1.8%] were attributed to fluoroquinolones including 6 cipro-, 4 moxi-, 1 levo-, and 1 gatifloxacin; average time to onset 4 days [range 1-39], with both hepatocellular and cholestatic enzyme patterns, seven with rash or fever, mortality limited to those with hepatocellular injury and jaundice; hepatic injury appeared to be class specific; no cases were attributed to gemifloxacin).
- Kilincalp S, Deveci M, Coban S, Basar O, Yuksel O. A new cause of acute hepatitis: gemifloxacin. Acta Gastroenterol Belg. 2012;75:460–1. [PubMed: 23402094](45 year old woman who was known to be HBsAg positive developed jaundice and pruritus on day 7 of a 10 day course of gemifloxacin [bilirubin 8.2 mg/dL, ALT 361 U/L, Alk P 276 U/L, HBV DNA negative], resolving within 4 weeks of stopping).
- Amitabh V, Singhal A, Kumar S, Patel N, Rizvi YS, Mishra P. Efficacy and safety of oral gemifloxacin for the empirical treatment of pneumonia. Lung India. 2012;29:248–53. [PMC free article: PMC3424864] [PubMed: 22919164](Open label study of 105 patients with pneumonia treated with gemifloxacin for 5-7 days found no overall change in ALT, AST or Alk P levels; one patient developed elevated Alk P levels, but it was considered unrelated to the drug).
- Kwon H, Lee SH, Kim SE, Lee JH, Jee YK, Kang HR, Park BJ, et al. Spontaneously reported hepatic adverse drug events in Korea: multicenter study. J Korean Med Sci. 2012;27:268–73. [PMC free article: PMC3286773] [PubMed: 22379337](Summary of 2 years of adverse event reporting in Korea; of 9360 reports, 567 were liver related, including 29 [5.1%] attributed to quinolones).
- Harr T, French LE. Stevens-Johnson syndrome and toxic epidermal necrolysis. Chem Immunol Allergy. 2012;97:149–66. [PubMed: 22613860](Review of the clinical features, epidemiology, genetics and pathogenesis of SJS and TEN).
- Patel TK, Barvaliya MJ, Sharma D, Tripathi C. A systematic review of the drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Indian population. Indian J Dermatol Venereol Leprol. 2013;79:389–98. [PubMed: 23619444](Systematic review of 10 case series of SJS/TEN from India identified 352 cases, among which 342 implicated a medication with most common being antimicrobials [37%], anticonvulsants [16%] and NSAIDs [16%]; fluoroquinolones accounted for 33 cases [10%], 4 of which were due to ciprofloxacin, 1 to ofloxacin and 1 to levofloxacin).
- Alshammari TM, Larrat EP, Morrill HJ, Caffrey AR, Quilliam BJ, LaPlante KL. Risk of hepatotoxicity associated with fluoroquinolones: a national case-control safety study. Am J Health Syst Pharm. 2014;71:37–43. [PubMed: 24352180](Retrospective analysis of Veterans Affairs patients receiving a fluoroquinolone [n=7862] found a higher relative risk of developing acute liver injury after receipt of ciprofloxacin compared to matched controls [adjusted odds ratio: OR=1.29], but not after receipt of levofloxacin [OR=1.16) or moxifloxacin [OR=0.98]).
- Arabyat RM, Raisch DW, McKoy JM, Bennett CL. Fluoroquinolone-associated tendon-rupture: a summary of reports in the Food and Drug Administration's adverse event reporting system. Expert Opin Drug Saf. 2015;14:1653–60. [PubMed: 26393387](Between 2004 and 2016, 2495 instances of spontaneous tendon rupture attributed to fluoroquinolones were reported to the FDA MedWatch system, including 1555 [62%] linked to levofloxacin, 606 [24%] ciprofloxacin, 230 [9%] moxifloxacin, but only 4 [0.2%] to gemifloxacin).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury seen over a ten year period at 8 US medical centers, 323 [36%] were attributed to antibiotics of which 38 were due to fluoroquinolones including 16 to ciprofloxacin, 13 levofloxacin, 8 moxifloxacin and 1 gatifloxacin, but none were attributed to gemifloxacin).
- Goldberg DS, Forde KA, Carbonari DM, Lewis JD, Leidl KB, Reddy KR, Haynes K, et al. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology 2015; 148: 1353-61. e3. [PMC free article: PMC4446162] [PubMed: 25733099](Analysis of Kaiser Permanente health care database from 2004 to 2011 identified 62 patients with suspected acute liver failure, 32 [52%] of whom had a presumed drug etiology, the most common being acetaminophen [18: 56%] and various herbal products [5: 16%], with single instances attributed to imatinib, simvastatin, leflunomide, isoniazid and valproate, but none to ciprofloxacin or other fluoroquinolones).
- Bonkovsky HL, Kleiner DE, Gu J, Odin JA, Russo MW, Navarro VM, Fontana RJ, Ghabril MS, et al. U.S. Drug Induced Liver Injury Network Investigators. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology. 2017;65:1267–77. [PMC free article: PMC5360519] [PubMed: 27981596](Among 363 patients with drug induced liver injury who underwent liver biopsy, 26 [7%] had bile duct loss of whom 94% developed evidence of chronic liver injury suggestive of vanishing bile duct syndrome, 2 of which were due to fluoroquinolones, 1 to moxifloxacin and 1 levofloxacin).
- Comparison table: some systemic fluoroquinolones. Med Lett Drugs Ther. 2018;60:e57–e58. [PubMed: 29635268](Table comparing 4 fluoroquinolones [cipro-, levo-, dela- and moxifloxacin] mentions that ALT and AST elevations are a class adverse event).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Gemifloxacin for the treatment of respiratory tract infections: in vitro susceptibility, pharmacokinetics and pharmacodynamics, clinical efficacy, and safety.[Pharmacotherapy. 2005]Review Gemifloxacin for the treatment of respiratory tract infections: in vitro susceptibility, pharmacokinetics and pharmacodynamics, clinical efficacy, and safety.Bhavnani SM, Andes DR. Pharmacotherapy. 2005 May; 25(5):717-40.
- Review Gemifloxacin: a new, potent fluoroquinolone for the therapy of lower respiratory tract infections.[Expert Rev Anti Infect Ther. 2...]Review Gemifloxacin: a new, potent fluoroquinolone for the therapy of lower respiratory tract infections.File TM Jr, Tillotson GS. Expert Rev Anti Infect Ther. 2004 Dec; 2(6):831-43.
- Review Gemifloxacin.[Drugs. 2000]Review Gemifloxacin.Lowe MN, Lamb HM. Drugs. 2000 May; 59(5):1137-47; discussion 1148.
- Review A new respiratory fluoroquinolone, oral gemifloxacin: a safety profile in context.[Int J Antimicrob Agents. 2004]Review A new respiratory fluoroquinolone, oral gemifloxacin: a safety profile in context.Ball P, Mandell L, Patou G, Dankner W, Tillotson G. Int J Antimicrob Agents. 2004 May; 23(5):421-9.
- Efficacy and safety of oral gemifloxacin for the empirical treatment of pneumonia.[Lung India. 2012]Efficacy and safety of oral gemifloxacin for the empirical treatment of pneumonia.Amitabh V, Singhal A, Kumar S, Patel N, Rizvi YS, Mishra P. Lung India. 2012 Jul; 29(3):248-53.
- Gemifloxacin - LiverToxGemifloxacin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...