NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Goserelin is a parenterally administered, gonadotropin releasing hormone (GnRH) agonist which causes an inhibition of estrogen and androgen production and is used predominantly to treat prostate cancer. Goserelin has been associated with a modest rate of serum enzyme elevations during therapy, but has not been convincingly linked to instances of clinically apparent acute liver injury.
Background
Goserelin (goe" se rel' in) is a synthetic decapeptide analogue of gonadotropin releasing hormone that acts as a partial agonist of the gonadotropin receptors in the pituitary that regulate luteinizing hormone (LH) and follicle stimulating hormone (FSH) secretion. These gonadotropins cause production and secretion of testosterone by the male testes and estrogen by the female ovaries. The continued receptor occupancy by goserelin, however, ultimately causes a down-regulation of production of LH and FSH and a resultant decrease in testosterone and estrogen levels. Goserelin, alone or in combination with other antiandrogens, has been found to be palliative in advanced prostate cancer. Goserelin was approved for use in the United States in 1989 and is still widely used, being considered important adjuvant therapy in management of advanced prostate cancer. Goserelin is also approved for use in advanced breast cancer and for several hormonally sensitive benign conditions such as endometriosis. The GnRH agonists have also been used off label for precocious puberty, infertility, and as a part of gender affirming therapy. Goserelin is available under the brand name Zoladex in solution for administration subcutaneously as implants every 4 (3.6 mg) or 12 (10.8 mg) weeks. Goserelin and the other GnRH analogues cause a profound hypogonadism ("chemical castration") and its common side effects are typical of androgen deprivation, including hot flashes, loss of libido, erectile dysfunction, depression, nausea, diarrhea, weight gain and fluid retention. Rare, but potentially severe adverse events can include immediate hypersensitivity reactions, pituitary apoplexy and, with long term use, weight gain, metabolic changes, diabetes and osteoporosis.
Hepatotoxicity
Goserelin has been associated with mild serum enzyme elevations during therapy in 3% to 5% of patients, but values above 3 times the upper limit of normal are rare, being reported in less than 1% of recipients. The serum enzyme elevations during goserelin therapy have generally been transient and asymptomatic, resolving even with drug continuation and rarely requiring dose modification or discontinuation. Despite use for several decades, goserelin has been linked to only a single, and not entirely convincing, case of clinically apparent liver injury. Routine monitoring of patients for liver test abnormalities is not recommended.
Likelihood score: D (possible, rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of liver test abnormalities during goserelin therapy is not known. Goserelin is a short peptide and is metabolized locally in many tissues. It is not metabolized to an appreciable extent by the hepatic cytochrome P450 system and has not been associated with significant drug-drug interactions. Some serum enzyme elevations may be caused by nonalcoholic fatty liver arising because of weight gain or metabolic changes caused by the androgen deprivation state induced by the GnRH agonist.
Outcome and Management
The serum enzyme elevations that occur on goserelin therapy usually do not require dose adjustment or drug discontinuation, but should lead to a search for other causes of liver disease. There is no evidence to indicate that there is cross sensitivity to liver injury among the various GnRH analogues.
Drug Class: Antineoplastic Agents, GnRH Analogues
Other Drugs in the Subclass, GnRH Analogues: Degarelix, Histrelin, Leuprolide, Relugolix, Triptorelin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Goserelin – Generic, Zoladex®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Goserelin | 65807-02-5 | C59-H84-N18-O14 |
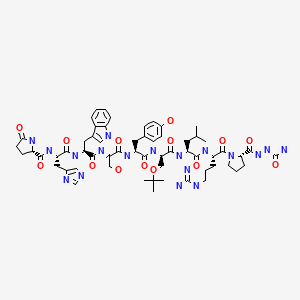
|
ANNOTATED BIBLIOGRAPHY
References updated: 28 May 2023
Abbreviations: FSH, follicle stimulating hormone; GnRH, gonadotropin releasing hormone; LH, luteinizing hormone; PSA, prostate specific antigen.
- Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 699.(Expert review of hepatotoxicity published in 1999; goserelin and other GnRH analogues are not discussed).
- Chitturi S, Farrell GC. Estrogen receptor antagonists. Adverse effects of hormones and hormone antagonists on the liver. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 610-2.(Review of hepatotoxicity of hormonal products; does not discuss the GnRH analogues such as goserelin).
- Levin ER, Vitek WS, Hammes SR. Estrogens, progestins, and the female reproductive tract. In, Brunton LL, Halal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 803-31.(Textbook of pharmacology and therapeutics).
- Snyder PJ. Androgens and the male reproductive tract. In, Brunton LL, Halal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 833-43.(Textbook of pharmacology and therapeutics).
- Isaacs C, Wellstein A, Riegel AT. Hormones and related agents in the therapy of cancer. In, Brunton LL, Halal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1237-47.(Textbook of pharmacology and therapeutics).
- Peeling WB. Phase III studies to compare goserelin (Zoladex) with orchiectomy and with diethylstilbestrol in treatment of prostatic carcinoma. Urology. 1989;33(5) Suppl:45–52. [PubMed: 2523611](In two large multicenter trials comparing goserelin to orchiectomy [358 patients] and goserelin to DES [250 patients], survival was similar with either treatment and goserelin had fewer side effects than DES; no mention of ALT elevations or hepatotoxicity).
- Miller RM, Frank RA. Zoladex (goserelin) in the treatment of benign gynaecological disorders: an overview of safety and efficacy. Br J Obstet Gynaeco. 1992;99 Suppl 7:37–41. [PubMed: 1532509](Review of the efficacy and safety of goserelin in 866 patients with various benign disorders mentions that there were no deaths, no unexpected severe adverse events, and "no clinically relevant effect on the usual biochemical parameters such as urea, electrolytes and liver-function tests").
- Maillefert JF, Sibilia J, Kuntz JL, Tavernier C. Gonadotrophin-releasing hormone agonists induce osteoporosis. Br J Rheumatol. 1994;33:1199–200. [PubMed: 8000764](Two men with prostate cancer, ages 58 and 69 years, were treated with leuprolide for 3 years and triptorelin for 9 months when they presented with back pain and vertebral fractures which were not present on pretreatment imaging).
- Vogelzang NJ, Chodak GW, Soloway MS, Block NL, Schellhammer PF, Smith JA Jr, Caplan RJ, et al. Goserelin versus orchiectomy in the treatment of advanced prostate cancer: final results of a randomized trial. Zoladex Prostate Study Group. Urology. 1995;46:220–6. [PubMed: 7624991](Among 283 patients with prostate cancer treated with goserelin or orchiectomy, objective responses and survival were the same in the two groups and the toxicity of goserelin "was decidedly minor" and not particularly different from that after surgery; no mention of ALT elevations or hepatotoxicity).
- Dijkman GA, Debruyne FM, Fernandez del Moral P, Plasman JW, Hoefakker JW, Idema JG, Sykes M. A randomised trial comparing the safety and efficacy of the Zoladex 10.8-mg depot, administered every 12 weeks, to that of the Zoladex 3.6-mg depot, administered every 4 weeks, in patients with advanced prostate cancer. Eur Urol. 1995;27:43–6. [PubMed: 7744141](Comparison safety and efficacy of monthly [3.6 mg] vs 3-monthly [10.8 mg] depot injections of goserelin in 80 patients with advanced prostate cancer found no difference in testosterone responses or side effects).
- Debruyne FM, Dijkman GA, Lee DC, Witjes WP, del Moral F, Karthaus HF, van der Mejden AP, et al. A new long acting formulation of the luteinizing hormone-releasing hormone analogue goserelin: results of studies in prostate cancer. J Urol. 1996;155:1352–4. [PubMed: 8632572](Comparison of goserelin depot subcutaneous injections every 4 weeks [3.6 mg] or every 12 weeks [10.8 mg] found no differences in efficacy or safety; no mention of ALT elevations or hepatotoxicity).
- Thorpe SC, Azmatullah S, Fellows GJ, Gingell JC, O'Boyle PJ. A prospective, randomised study to compare goserelin acetate (Zoladex) versus cyproterone acetate (Cyprostat) versus a combination of the two in the treatment of metastatic prostatic carcinoma. Eur Urol. 1996;29:47–54. [PubMed: 8821690](Among 525 patients with advanced prostate cancer treated with goserelin, cyproterone or both, the combination had no advantage in prolonging survival, but did reduce side effects of hot flashes; no mention of ALT elevations or hepatotoxicity).
- Hands KE, Alvarez A, Bruder JM. Gonadotropin-releasing hormone agonist-induced pituitary apoplexy in treatment of prostate cancer: case report and review of literature. Endocr Pract. 2007;13:642–6. [PubMed: 17954421](Review of 7 cases of pituitary apoplexy occurring after initiation of GnRH agonist therapy for prostate cancer).
- Guerra Y, Lacuesta E, Marquez F, Raksin PB, Utset M, Fogelfeld L. Apoplexy in non functioning pituitary adenoma after one dose of leuprolide as treatment for prostate cancer. Pituitary. 2010;13:54–9. [PubMed: 19842040](60 year old man with prostate cancer developed headaches and neurologic symptoms within 24 hours of a first injection of leuprolide, and subsequent evaluation revealed a previously unsuspected non-functioning pituitary adenoma).
- Masuda N, Iwata H, Rai Y, Anan K, Takeuchi T, Kohno N, Takei H, et al. Monthly versus 3-monthly goserelin acetate treatment in pre-menopausal patients with estrogen receptor-positive early breast cancer. Breast Cancer Res Treat. 2011;126:443–51. [PubMed: 21221770](Among 170 patients with estrogen receptor positive early breast cancer treated with goserelin every 4 [3.6 mg] or every 12 [10.8 mg] weeks, there were no differences in markers of efficacy, safety or tolerability and no drug related serious adverse events; no mention of ALT elevations).
- Duburque C, Bonnal JL, Gosset P, Lucidarme D. Prog Urol. 2012;22:610–2. [Could gosereline acetate induce autoimmune-like hepatitis?] French. [PubMed: 22920341](59 year old man with prostate cancer and heavy alcohol intake [80 gm daily] developed jaundice 6 weeks after stopping bicalutamide and 10 days after a 2nd every-12-week injection of goserelin [bilirubin 21.3 mg/dL, ALT 50 times ULN, Alk P 2 times ULN, ANA 1:640, MCV 105], resolving 4 months after onset).
- Van Poppel H, Klotz L. Gonadotropin-releasing hormone: an update review of the antagonists versus agonists. Int J Urol. 2012;19:594–601. [PubMed: 22416801](Review of androgen deprivation therapy for prostate cancer using GnRH agonists and antagonists stressing the more rapid onset of action and similar if not better safety profile of GnRH antagonists).
- Walker LM, Tran S, Robinson JW. Luteinizing hormone--releasing hormone agonists: a quick reference for prevalence rates of potential adverse effects. Clin Genitourin Cancer. 2013;11:375–84. [PubMed: 23891497](Systematic review of adverse event profile of long term use of GnRH agonists, which mostly relate to the hormonal changes that occur: hot flashes, gynecomastia, genital shrinkage, hair loss, osteoporosis, mild anemia, hyperglycemia, increased weight, loss of skeletal muscle mass, emotional lability, depression, loss of sexual desire and erectile dysfunction; no mention of ALT elevations or hepatotoxicity).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none of the 96 were attributed to goserelin or any of the GnRH analogues).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e.7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to goserelin or any of the GnRH analogues).
- Noguchi S, Kim HJ, Jesena A, Parmar V, Sato N, Wang HC, Lokejaroenlarb S, et al. Phase 3, open-label, randomized study comparing 3-monthly with monthly goserelin in pre-menopausal women with estrogen receptor-positive advanced breast cancer. Breast Cancer. 2016;2:771–9. [PMC free article: PMC4999470] [PubMed: 26350351](Among 222 premenopausal women with breast cancer treated with tamoxifen and goserelin [every 1 or 3 months], adverse events included hot flush [17%], headache [6%], back pain [6%], and nausea [5%]; no mention of ALT elevations or hepatotoxicity).
- Bolton EM, Lynch TH. Are all gonadotropin-releasing hormone agonists equivalent for the treatment of prostate cancer? A systematic review. BJU Int. 2018;122(3):371–83. [PubMed: 29438592](Systematic review of literature on relative efficacy and safety of different GnRH agonists, indicates that there is little evidence of superiority of any of the four, largely because of lack of adequately powered, controlled studies comparing them; no mention of ALT elevations or hepatotoxicity).
- Kim JY, Im SA, Jung KH, Ro J, Sohn J, Kim JH, Park YH, et al. breast cancer committee of Korean Cancer Study Group (KCSG). Fulvestrant plus goserelin versus anastrozole plus goserelin versus goserelin alone for hormone receptor-positive, HER2-negative tamoxifen-pretreated premenopausal women with recurrent or metastatic breast cancer (KCSG BR10-04): a multicentre, open-label, three-arm, randomised phase II trial (FLAG study). Eur J Cancer. 2018;103:127–136. [PubMed: 30223226](Among 283 Korean premenopausal women with metastatic breast cancer treated with goserelin alone or combined with either fluvestrant or anastrozole, time to progression was longest with fluvestrant and goserelin, although adverse event rates were similar in all three groups: no mention of ALT levels or hepatotoxicity).
- Cornford P, Jefferson K, Cole O, Gilbody J. Effects of initiating or switching to a six-monthly triptorelin formulation on prostate cancer patient-healthcare interactions and hospital resource use: a real-world, retrospective, non-interventional study. Oncol Ther. 2018;6:173–187. [PMC free article: PMC7359994] [PubMed: 32700031](Among 41 adults with advanced prostate cancer who were switched from every 1 or 3 monthly GnRH regimen to 6 monthly triptorelin, healthcare visits, injections and PSA testing were less as were adverse side effects including fatigue [12% vs 26%], urinary frequency [9% vs 32%], and bone pain [7% vs 14%]; no mention of ALT elevations or hepatotoxicity).
- Sun Y, Xie L, Xu T, Jakobsen JS, Han W, Sørensen PS, Wang X. Efficacy and safety of degarelix in patients with prostate cancer: Results from a phase III study in China. Asian J Urol. 2020;7:301–308. [PMC free article: PMC7385516] [PubMed: 32742930](Among 283 Chinese men with prostate cancer treated with goserelin or degarelix for 12 months, suppression of serum testosterone to castrate levels was more rapid with degarelix but ultimate rates achieved were similar in both groups, and while total adverse events were fewer with goserelin alone [59% vs 76%], serious adverse event rates were similar in the two groups and there was no mention of ALT levels or hepatotoxicity).
- Bahl A, Rajappa S, Rawal S, Bakshi G, Murthy V, Patil K. A review of clinical evidence to assess differences in efficacy and safety of luteinizing hormone-releasing hormone (LHRH) agonist (goserelin) and LHRH antagonist (degarelix). Indian J Cancer. 2022;59 Supplement:S160–S174. [PubMed: 35343199](Systematic review of studies comparing the efficacy and safety of the 3 GnRH analogues used in therapy of prostate cancer identified 12 studies which showed overall no differences in efficacy in reducing serum testosterone levels and, in 4 studies reporting data on safety, similar degrees of tolerance and rates of adverse events: no mention of ALT elevations or hepatotoxicity).
- Lambertini M, Boni L, Michelotti A, Magnolfi E, Cogoni AA, Mosconi AM, Giordano M, et al. GIM study group. Long-term outcomes with pharmacological ovarian suppression during chemotherapy in premenopausal early breast cancer patients. J Natl Cancer Inst. 2022;114:400–408. [PMC free article: PMC8902441] [PubMed: 34850043](Among 281 women with early onset breast cancer treated with chemotherapy with or without a GnRH analogue to preserve ovarian function, disease-free and overall survival were similar in the two groups and those given the GnRH analogue were slightly more likely to have a successful pregnancy during follow up [6.5% vs 3.2%]; no mention of other adverse events, ALT levels or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Leuprolide.[LiverTox: Clinical and Researc...]Review Leuprolide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Gonadotropin Releasing Hormone (GnRH) Analogues.[LiverTox: Clinical and Researc...]Review Gonadotropin Releasing Hormone (GnRH) Analogues.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Degarelix.[LiverTox: Clinical and Researc...]Review Degarelix.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Histrelin.[LiverTox: Clinical and Researc...]Review Histrelin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Gonadotropin-releasing hormone agonists in prostate cancer: A comparative review of efficacy and safety.[Indian J Cancer. 2022]Review Gonadotropin-releasing hormone agonists in prostate cancer: A comparative review of efficacy and safety.Raja T, Sud R, Addla S, Sarkar KK, Sridhar PS, Talreja V, Jain M, Patil K. Indian J Cancer. 2022 Mar; 59(Supplement):S142-S159.
- Goserelin - LiverToxGoserelin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...