NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Lemborexant is an orexin receptor antagonist used for the treatment of insomnia and sleep disorders. Lemborexant therapy is associated with rare occurrence of transient serum enzyme elevations, but has not been implicated in cases of clinically apparent liver injury.
Background
Lemborexant (lem” boe rex' ant) is an orexin receptor antagonist which is used to treat insomnia. Central nervous system neurons with orexin receptors are involved in wakefulness and are inactive during sleep. Engagement of the orexin receptor results in wakefulness, and loss of the receptor can result in excessive daytime sleepiness and narcolepsy. Inhibition of orexin receptor signaling using lemborexant has been shown to shorten the time to falling asleep and to prolong sleep in patients with sleep-onset and sleep-maintenance insomnia. Lemborexant was approved for use in the United States in 2019, the second such orexin based drug approved for chronic insomnia (the first being suvorexant). Lemborexant is available in tablets of 5 and 10 mg under the brand name Dayvigo. The recommended dose is 5 mg taken orally within 30 minutes of bedtime. The dose can be increased to 10 mg. Side effects are few, but may include daytime somnolence, fatigue, dizziness, headache and vivid or abnormal dreams. Rare side effects include sleep paralysis, cataplexy, nightmares, excessive daytime sleepiness, worsening of depression and suicidal ideation and behaviors. Both lemborexant and suvorexant are classified as a Schedule 4 controlled substances, meaning that they have a low potential for abuse or dependence but have an approved medical use.
Hepatotoxicity
In several clinical trials, lemborexant was found to be well tolerated, with serum ALT elevations above 3 times the upper limit of normal in less than 1% of treated subjects and with similar rates in placebo recipients. The elevations were transient and asymptomatic, none required dose modification or discontinuation, and none were associated with a simultaneous elevation in serum bilirubin. Thus, in the registration trials of lemborexant, there were no reports of clinically apparent liver injury. Lemborexant has been available for a limited period of time, but has yet to be implicated in causing clinically apparent liver injury with its more widespread clinical use.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which lemborexant might cause liver injury is unknown. Lemborexant is metabolized by the cytochrome P450 system (predominantly CYP3A4) and the dose may need to be altered in patients taking strong inhibitors (decreasing dose) or inducers (increasing dose) of the enzymes. The relative lack of serious liver related adverse events is probably due to the low doses used and its uncommon, intermittent use.
Drug Class: Sedatives and Hypnotics
Other Related Drug for Insomnia: Suvorexant
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Lemborexant – Dayvigo®
DRUG CLASS
Sedatives and Hypnotics
Product labeling at DailyMed, National Library of Medicine, NIH
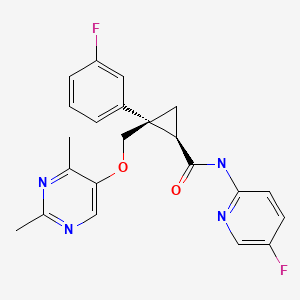
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Lemborexant | 1369764-02-2 | C22-H20-F2-N4-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 25 September 2021
- Zimmerman HJ. Unconventional drugs. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 731-4.(Expert review of hepatotoxicity published in 1999; lemborexant and the orexin receptor antagonists are not discussed).
- Larrey D, Ripault MP. Anxiolytic agents. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 455-6.(Review of hepatotoxicity of hypnotics and sedatives discusses benzodiazepines, buspirone and valerian, all of which have been linked to rare cases of liver injury; suvorexant and orexin receptor antagonists are not discussed).
- Mihic SJ, Mayfield J, Harris RA. Hypnotics and sedatives. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 339-354.(Textbook of pharmacology and therapeutics).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2019/212028Orig1s000MultidisciplineR.pdf. (FDA website with product labels and multiple disciplinary review that supported approval of lemborexant in the US, mentions that there were no deaths, no evidence of abuse or diversion, no hepatic serious adverse events, no discontinuations for hepatic events, and no significant shift or change in serum enzyme levels during therapy, no instance of ALT elevation with jaundice, and in registration trials, ALT elevations of more than 3 times ULN arose in 0.6% of lemborexant and 0.6% of placebo recipients). - Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 82 [9%] were attributed to agents active in the central nervous system, but none were sedatives or sleeping aids).
- Drugs for insomnia. Med Lett Drugs Ther. 2015;57(1472):95–8. [PubMed: 26147892](Concise review of the mechanism of action, efficacy, safety and costs of drugs for insomnia including benzodiazepine receptor agonists, benzodiazepines, melatonin receptor agonists, orexin receptor antagonists such as suvorexant and other agents including nonprescription and herbal products; no mention of ALT elevations or hepatotoxicity).
- Murphy P, Moline M, Mayleben D, Rosenberg R, Zammit G, Pinner K, Dhadda S, et al. Lemborexant, a dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: results from a Bayesian, adaptive, randomized, double-blind, placebo-controlled study. J Clin Sleep Med. 2017;13:1289–1299. [PMC free article: PMC5656478] [PubMed: 29065953](Among 291 adults with insomnia treated with lemborexant [1, 2.5, 5, 10, 15 or 25 mg] vs placebo once nightly for 15 days, doses of 2.5 to 10 mg yielded the optimal balance of efficacy vs next morning residual sleepiness [~3% vs 22% at highest doses], and there were “no clinically important differences” between drug and placebo treatment in blood chemistry results).
- Rosenberg R, Murphy P, Zammit G, Mayleben D, Kumar D, Dhadda S, Filippov G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2:e1918254. [PMC free article: PMC6991236] [PubMed: 31880796](Among 1006 older adults with insomnia, treated with study drugs once nightly for 1 month, time to sleep onset and sleep maintenance were improved more with lemborexant [5 or 10 mg] than zolpidem or placebo, and adverse events were largely mild-to-moderate, with no treatment related serious adverse events, no deaths and “no notable findings were reported for clinical laboratory tests”).
- Scott LJ. Lemborexant: first approval. Drugs. 2020;80:425–432. [PubMed: 32096020](Review of the mechanism of action, history of development, pharmacology, clinical efficacy and safety of lemborexant, shortly after its approval for treatment of insomnia in the US, does not mention ALT elevations or hepatotoxicity).
- Lemborexant (Dayvigo) for insomnia. Med Lett Drugs Ther. 2020;62(1601):97–100. [PubMed: 32724021](Concise review of the mechanism of action, clinical efficacy, safety and costs of lemborexant, mentions side effects of somnolence, headaches, abnormal dreams, depression, suicidal ideation and sleep paralysis and cataplexy, but no mention of ALT elevations or hepatotoxicity).
- Kärppä M, Yardley J, Pinner K, Filippov G, Zammit G, Moline M, Perdomo C, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43:zsaa123. [PMC free article: PMC7487867] [PubMed: 32585700](Among 949 adults with insomnia treated with lemborexant [5 or 10 mg] vs placebo for 6 months, improvements in time to sleep onset and sleep maintenance were greater with lemborexant and overall adverse event rates were similar, except for somnolence [4.1% and 8.6% vs 1.6%] and fatigue [3.8% and 3.5% vs 0.3%], and there were “no clinically important mean changes in … chemistry laboratory parameters” while the incidence of abnormal values was low and similar in the three groups).
- Muehlan C, Vaillant C, Zenklusen I, Kraehenbuehl S, Dingemanse J. Clinical pharmacology, efficacy, and safety of orexin receptor antagonists for the treatment of insomnia disorders. Expert Opin Drug Metab Toxicol. 2020;16:1063–1078. [PubMed: 32901578](Review of the role of orexin in sleep and wakefulness, and the mechanism of action, pharmacology, clinical efficacy and safety of orexin receptor antagonists including suvo-, lembo-, darido- and selto-rexant, all of which have similar side effects but are generally “well tolerated”).
- Moline M, Thein S, Bsharat M, Rabbee N, Kemethofer-Waliczky M, Filippov G, Kubota N, et al. Safety and efficacy of lemborexant in patients with irregular sleep-wake rhythm disorder and Alzheimer's disease dementia: results from a phase 2 randomized clinical trial. J Prev Alzheimers Dis. 2021;8:7–18. [PubMed: 33336219](Among 62 adults with Alzheimer disease and irregular sleep-wake rhythm disorder treated with lemborexant [2.5, 5, 10 or 15 mg] vs placebo for 4 weeks, measures of restlessness and circadian rhythm variables improved with lemborexant, and there were no serious adverse events or treatment related adverse events leading to discontinuation; no mention of ALT or laboratory test results).
- Yardley J, Kärppä M, Inoue Y, Pinner K, Perdomo C, Ishikawa K, Filippov G, et al. Long-term effectiveness and safety of lemborexant in adults with insomnia disorder: results from a phase 3 randomized clinical trial. Sleep Med. 2021;80:333–342. [PubMed: 33636648](Among 949 adults with insomnia enrolled in a randomized, placebo-control trial of lemborexant for 6 months followed by open label drug for a total of 12 months, improvements in time to sleep onset, sleep maintenance and self-reported sleep quality were maintained, adverse event rates tended to decrease with continued therapy, and “there were no clinically significant findings for any…chemistry laboratory parameter”).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- [Preclinical and clinical efficacy of orexin receptor antagonist Lemborexant (Dayvigo(®)) on insomnia patients].[Nihon Yakurigaku Zasshi. 2021][Preclinical and clinical efficacy of orexin receptor antagonist Lemborexant (Dayvigo(®)) on insomnia patients].Koebisu M, Koyama N, Nishida M, Muramoto K. Nihon Yakurigaku Zasshi. 2021; 156(2):114-119.
- Improvement in fatigue and sleep measures with the dual orexin receptor antagonist lemborexant in adults with insomnia disorder.[Postgrad Med. 2022]Improvement in fatigue and sleep measures with the dual orexin receptor antagonist lemborexant in adults with insomnia disorder.Chepke C, Jain R, Rosenberg R, Moline M, Yardley J, Pinner K, Kumar D, Perdomo C, Filippov G, Atkins N, et al. Postgrad Med. 2022 Apr; 134(3):316-325. Epub 2022 Mar 20.
- Review Lemborexant, an orexin receptor antagonist sedative-hypnotic: Is it useful for insomnia in psychiatric disorders?[Australas Psychiatry. 2022]Review Lemborexant, an orexin receptor antagonist sedative-hypnotic: Is it useful for insomnia in psychiatric disorders?Keks NA, Hope J. Australas Psychiatry. 2022 Aug; 30(4):530-532. Epub 2022 May 1.
- Preclinical in vivo characterization of lemborexant (E2006), a novel dual orexin receptor antagonist for sleep/wake regulation.[Sleep. 2019]Preclinical in vivo characterization of lemborexant (E2006), a novel dual orexin receptor antagonist for sleep/wake regulation.Beuckmann CT, Ueno T, Nakagawa M, Suzuki M, Akasofu S. Sleep. 2019 Jun 11; 42(6).
- Review Review of the Efficacy and Safety of Lemborexant, a Dual Receptor Orexin Antagonist (DORA), in the Treatment of Adults With Insomnia Disorder.[Ann Pharmacother. 2022]Review Review of the Efficacy and Safety of Lemborexant, a Dual Receptor Orexin Antagonist (DORA), in the Treatment of Adults With Insomnia Disorder.Waters K. Ann Pharmacother. 2022 Feb; 56(2):213-221. Epub 2021 Jun 2.
- Lemborexant - LiverToxLemborexant - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...