NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Levodopa (L-Dopa) is an amino acid precursor of dopamine and is the most effective and commonly used drug in the treatment of Parkinson disease. Levodopa is usually combined with carbidopa, which is an inhibitor of L-amino acid decarboxylase, the plasma enzyme that metabolizes levodopa peripherally. Treatment with the combination of levodopa and carbidopa has been associated with mild and transient increases in serum enzymes in a proportion of patients and with very rare instances of clinically apparent acute liver injury.
Background
Levodopa (lee" voe doe' pa) is a derivative of phenylalanine and is a metabolic precursor of dopamine. Levodopa crosses the blood brain barrier where it is converted to dopamine by decarboxylation in the presynaptic terminals of dopaminergic neurons. After release, it is transported back into the dopaminergic terminals or is metabolized either by catechol-O-methyltransferase (COMT) or by monoamine oxidase (MAO). When given orally, levodopa is usually combined with carbidopa (kar” bi doe' pa), which is also a derivative of phenylalanine and is an inhibitor of L-amino acid decarboxylase, the plasma enzyme that metabolizes levodopa peripherally. Levodopa was approved for use in the United States in 1970 and the combination of levodopa and carbidopa in 1975. Levodopa, alone and in combination with carbodopa, remains a commonly used agent for Parkinson disease with more than 2 million prescriptions filled yearly in the United States. Current indications include therapy of symptomatic Parkinson disease as well as spastic disorders and extrapyramidal disorders due to medications. Levodopa is available as tablets with various fixed combination with carbidopa (10-100, 25-100 and 25-250) in generic forms and under the trade name Sinemet. The combination is typically given 3 to 4 times daily, although a controlled release form is available that allows for twice daily dosing. The optimal dose varies by patient; it is typically started at a low dose and increased based upon clinical response and tolerance. Side effects can include nausea, dyskinesias, hallucinations, confusion, postural hypotension, sedation, constipation, sleep disturbances, depression and hypersexuality – side effects that are common to all dopaminergic agents.
Hepatotoxicity
The combination of levodopa and carbidopa has been reported to cause serum aminotransferase elevations in up to 9% of patients, but these abnormalities are usually mild, asymptomatic and self-limiting. In rare instances, the aminotransferase elevations rise above 5 to 10 times the ULN and require discontinuation or dose adjustment. In addition, levodopa has been implicated in a small number of cases of clinically apparent, acute liver injury, but the clinical characteristics and typical pattern of enzyme elevations have not been characterized. There have been no published case reports of clinically apparent liver injury convincingly attributed to levodopa. In view of the long term and wide scale use of levodopa in Parkinson disease, clinically apparent liver injury with jaundice must be exceedingly rare.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The metabolism of levodopa and carbodopa is largely through peripheral or intracellular amino acid decarboxylases, and by catechol-O-methyltransferase and monoamine oxidase in the brain. They have minimal hepatic metabolism which perhaps accounts for its lack of hepatotoxicity.
Drug Class: Parkinson Disease Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Levodopa – L-Dopa®
Carbidopa – Lodosyn®
Carbidopa/Levodopa – Generic, Sinemet®
DRUG CLASS
Parkinson Disease Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Levodopa | 59-92-7 | C9-H11-N-O4 |
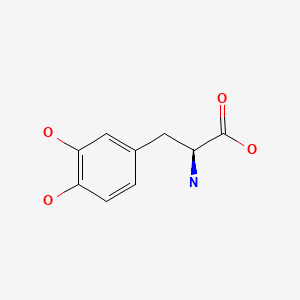
|
| Carbidopa | 38821-49-7 | C10-H14-N2-O4.H2-O |
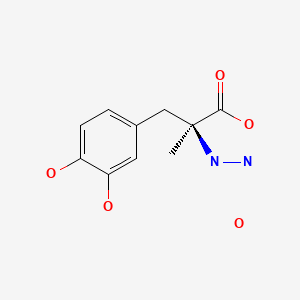
|
ANNOTATED BIBLIOGRAPHY
References updated: 25 October 2021
Abbreviations used: COMT, catechol O-methyltransferase; MAO, monoamine oxidase.
- Zimmerman HJ. Antiparkinsonism drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 715-7.(Expert review of hepatotoxicity published in 1999; among anticholinergic agents, "only trihexyphenidyl has been incriminated in hepatic injury"; other antiparkinsonism drugs discussed include levodopa, lergotrile [no longer available], pergolide and bromocriptine).
- Larrey D, Ripault MP. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier Inc, 2013, pp. 443-62.(Review of hepatotoxicity of agents acting on the central nervous system).
- Roberson ED. Treatment of central nervous system degenerative disorders. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 327-38.(Textbook of pharmacology and therapeutics).
- McDowell F. Symposium on levodopa in Parkinson's disease. Clinical and pharmacological aspects. Clinical laboratory abnormalities. Clin Pharmacol Ther. 1971;12:335–9. [PubMed: 4102803](Retrospective analysis of laboratory abnormalities arising in 974 patients with Parkinson disease treated with levodopa; AST elevations occurred in 9% of 5427 determinations, but were usually mild and transient returning to normal in 1-2 months without dose adjustment; AST levels rose to 1600 U/L in one patient who later died of complications of diabetes).
- Kanetaka T, Oda T. Toxic liver injuries. Acta Pathol Jpn. 1973;23:617–27. [PubMed: 4800729](In reviewing experience with drug induced liver disease, mentions one case attributed to levodopa therapy).
- Lieberman AN, Gopinathan G, Neophytides A, Goldstein M. Management of levodopa failures: the use of dopamine agonists. Clin Neuropharmacol. 1986;9:S9–21. [PubMed: 3297319](Among 278 patients with Parkinson disease failing to respond to levodopa and treated with a dopamine agonist, 61% improved but adverse events requiring discontinuation occurred in 46%; no discussion of hepatotoxicity).
- Lambert D, Waters CH. Comparative tolerability of the newer generation antiparkinsonian agents. Drugs Aging. 2000;16:55–65. [PubMed: 10733264](Review of mechanism of action, tolerability and safety of selegiline, pramipexole, ropinirole, tolcapone and entacapone in Parkinson disease).
- Levodopa + carbidopa + entacapone. Entacapone: a second look: new preparations. Parkinson's disease: a modest effect. Prescrire Int. 2005;14:51–4. [PubMed: 15875340](Review of risks and benefits of a fixed dose combination of levodopa, carbidopa and entacapone mentions that entacapone may cause cholestatic hepatitis and that is has not been shown to be more effective than bromocriptine).
- Jankovic J, Stacy M. Medical management of levodopa-associated motor complications in patients with Parkinson's disease. CNS Drugs. 2007;21:677–92. [PubMed: 17630819](Review of options for therapy of Parkinson disease in patients on long term levodopa manifesting fluctuating motor dysfunction).
- Lees AJ, Ratziu V, Tolosa E, Oertel WH. Safety and tolerability of adjunctive tolcapone treatment in patients with early Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007;78:944–8. [PMC free article: PMC2117861] [PubMed: 17098835](Controlled trial of tolcapone vs placebo combined with levodopa and carbidopa in 677 patients with early Parkinson disease; ALT or AST elevations occurred in 20% of placebo- vs 27% of tolcapone treated patients and were >3 times ULN in 1.2% [placebo] vs 1.8% [tolcapone], almost all during first 6 months; 1% of tolcapone treated patients stopped because of ALT elevations, but none developed jaundice or clinically apparent liver injury).
- Brooks DJ, Leinonen M, Kuoppamäki M, Nissinen H. Five-year efficacy and safety of levodopa/DDCI and entacapone in patients with Parkinson's disease. J Neural Transm. 2008;115:843–9. [PubMed: 18259682](Retrospective, pooled analysis of 5 controlled trials with 5 year extension phases that included 806 patients with Parkinson disease treated with entacapone added to levodopa/carbodopa; there were "few clinically significant changes in liver function tests" and none of the 478 serious adverse events that were reported were due to hepatotoxicity).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to agents used for Parkinson disease).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none of the 96 were attributed to an agent used to treat Parkinson disease).
- Drugs for Parkinson's disease. Treat Guidel Med Lett. 2013;11(135):101–6. [PubMed: 24165688](Concise review of recommendations for therapy of Parkinson disease with description of mechanisms of action, efficacy and adverse events).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to an agent to treat Parkinson disease).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury from the US enrolled in a prospective database between 2004 and 2012, none were attributed to an agent used to treat Parkinson disease).
- Drugs for Parkinson's disease. Med Lett Drugs Ther. 2017;59(1534):187–194. [PubMed: 29136401](Concise review of medications approved for use in Parkinson disease including levodopa/carbidopa, dopamine agonists, MAO-B inhibitors, anticholinergics, and COMT inhibitors, mentions hepatotoxicity of tolcapone but not of levodopa or any of the other adjunctive therapies: “Use of tolcapone requires written informed consent and monitoring of liver function every 2-4 weeks for the first 6 months of treatment and periodically thereafter. Serious hepatoxicity has not been reported with entacapone”).
- Margolesky J, Singer C. Extended-release oral capsule of carbidopa-levodopa in Parkinson disease. Ther Adv Neurol Disord. 2017;11:1756285617737728. [PMC free article: PMC5784558] [PubMed: 29399046](Review of the pharmacology, efficacy and safety of extended release carbidopa/levodopa in listing of reported adverse reactions there is no mention of serious hepatic events or ALT elevations).
- Drugs for Parkinson's disease. Med Lett Drugs Ther. 2021;63(1618):25–32. [PubMed: 33647001](Concise review of current medications approved for use in Parkinson disease including levodopa/carbidopa, dopamine agonists, COMT inhibitors, MAO-B inhibitors, anticholinergics, and istradefylline, mentions hepatotoxicity of tolcapone but not of levodopa or any of the adjunctive therapies).
- Hauser RA, Hattori N, Fernandez H, Isaacson SH, Mochizuki H, Rascol O, Stocchi F, et al. Efficacy of istradefylline, an adenosine A2A receptor antagonist, as adjunctive therapy to levodopa in Parkinson's disease: a pooled analysis of 8 phase 2b/3 trials. J Parkinsons Dis. 2021;11:1663–75. [PMC free article: PMC8609697] [PubMed: 34486986](In a pooled analysis of 8 randomized placebo-controlled trials of istradefylline in 2719 patients with Parkinson disease and motor complications, while adverse event rates were similar in the 3 groups [71% and 70% vs 65%] except for dyskinesia [16% and 18% vs 10%], and “no clinically meaningful changes in laboratory parameters…were observed” in any group including those on levodopa/carbidopa alone).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Parkinson Disease Agents.[LiverTox: Clinical and Researc...]Review Parkinson Disease Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Inhibition of decarboxylase and levels of dopa and 3-O-methyldopa: a comparative study of benserazide versus carbidopa in rodents and of Madopar standard versus Madopar HBS in volunteers.[Eur Neurol. 1987]Inhibition of decarboxylase and levels of dopa and 3-O-methyldopa: a comparative study of benserazide versus carbidopa in rodents and of Madopar standard versus Madopar HBS in volunteers.Da Prada M, Kettler R, Zürcher G, Schaffner R, Haefely WE. Eur Neurol. 1987; 27 Suppl 1:9-20.
- Protective effects of L-dopa and carbidopa combined treatments on human catecholaminergic cells.[DNA Cell Biol. 2012]Protective effects of L-dopa and carbidopa combined treatments on human catecholaminergic cells.Colamartino M, Padua L, Meneghini C, Leone S, Cornetta T, Testa A, Cozzi R. DNA Cell Biol. 2012 Nov; 31(11):1572-9. Epub 2012 Sep 28.
- In vivo protective effect of Uridine, a pyrimidine nucleoside, on genotoxicity induced by Levodopa/Carbidopa in mice.[Food Chem Toxicol. 2015]In vivo protective effect of Uridine, a pyrimidine nucleoside, on genotoxicity induced by Levodopa/Carbidopa in mice.Orenlili Yaylagul E, Cansev M, Celikler Kasimogullari S. Food Chem Toxicol. 2015 Aug; 82:36-41. Epub 2015 May 11.
- Review Determination of levodopa by chromatography-based methods in biological samples: a review.[Anal Sci. 2022]Review Determination of levodopa by chromatography-based methods in biological samples: a review.Jiang R, Yang J, Mei S, Zhao Z. Anal Sci. 2022 Aug; 38(8):1009-1017. Epub 2022 Jun 17.
- Levodopa - LiverToxLevodopa - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...