NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Naloxone is an opiate antagonist which is used intravenously in emergency situations to reverse the respiratory depression caused by overdoses of heroin, morphine or other opioids. Naloxone has not been linked to serum enzyme elevations during therapy or to clinically apparent liver injury.
Background
Naloxone (nal ox’ one) is a derivative of the natural plant alkaloid thebaine and is similar to oxymorphone. Naloxone, however, is a competitive antagonist of the opiate receptors and has particularly high affinity for the μ opiate receptor and can displace morphine and other full agonists, thereby reversing their effects. Naloxone causes rapid onset of withdrawal symptoms in opioid dependent persons and is generally used in emergency rooms to treat the respiratory depression caused by opioid overdose or postoperatively to reverse opioid anesthetic effects. Naloxone has sometimes been provided to emergency medical personnel and police and fire personnel for use onsite in emergency situations. Naloxone was first approved for use in the United States in 1971. It remains available as a solution for injection in generic forms and under the brand name Narcan, typically in concentrations of 0.4 mg/mL to be given intramuscularly or intravenously. It is also available as a nasal spray in solution of 4 mg/0.1mL. The usual initial dose in adults with suspected opioid overdose is 0.4 to 2.0 mg. Naloxone is also available in fixed combination with buprenorphine for sublingual use (Suboxone and generic) and with pentazocine for oral use. Naloxone is poorly absorbed by the sublingual or oral route and is added to discourage the intravenous or parenteral administration of buprenorphine or pentazocine. Side effects of naloxone in opioid dependent persons include mood changes, sweating, anxiety, restlessness, trembling, dizziness, flushing, headache, nausea, vomiting, cardiac tachyarrhythmias, seizures, chest pain and acute pulmonary edema—symptoms of acute opioid withdrawal. Naloxone has minimal effects in persons not taking opioids. Naloxone is not a controlled substance, but its use is sometimes restricted to medical staff trained in emergency medicine or anesthesia.
Hepatotoxicity
Therapy with naloxone has not been linked to serum enzyme elevations or to idiosyncratic acute, clinically apparent liver injury. Patients with opioid overdose often have underlying chronic liver diseases such as alcoholic liver disease, hepatitis B or C, but treatment with naloxone does not appear to exacerbate those conditions. Naloxone is extensively metabolized in the liver, but largely by conjugation with glucuronide followed by its urinary excretion.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Drug Class: Opioid Antagonists; see also Substance Abuse Treatment Agents
Other Drugs in the Class: Nalmefene; Naloxegol, Naltrexone
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Naloxone – Generic, Narcan®
DRUG CLASS
Opioid Antagonists
Product labeling at DailyMed, National Library of Medicine, NIH
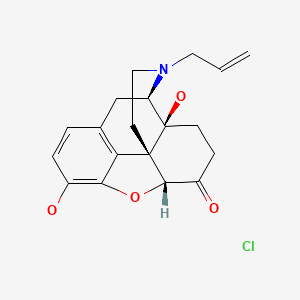
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Naloxone | 357-08-4 | C19-H21-N-O4.Cl-H |
|
ANNOTATED BIBLIOGRAPHY
References updated: 24 March 2020
- Zimmerman HJ. Narcotic analgesics. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 710-11.(Expert review of hepatotoxicity published in 1999; mentions that trials of naltrexone have reported serum aminotransferase elevations in up to 30% of recipients, an effect that appeared to be partially dose dependent; naloxone not discussed).
- Larrey D, Ripault MP. Illegal and recreational compounds. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 456-7.(Review of hepatotoxicity discusses buprenorphine, an orally available morphine analogue, which has been linked to cases of severe acute liver injury, usually as a result of intravenous administration; naloxone not discussed).
- Yaksh TL, Wallace MS. Opioids, analgesia, and pain management. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 355-86.(Textbook of pharmacology and therapeutics).
- Wiesen RL, Rich CR, Wang RI, Stockdale SL. The safety and value of naloxone as a therapeutic aid. Drug Alcohol Depend. 1977;2:123–30. [PubMed: 858271](Among 343 subjects with suspected opioid dependence given intravenous naloxone to assess the presence and degree of dependence, no patient developed a serious adverse event; no mention of liver injury or ALT levels).
- Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, Eisenberg HM, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–11. [PubMed: 2278545](Among 487 patients with acute spinal cord injury treated with intravenous, high dose methylprednisolone, naloxone or placebo, side effects and complications were comparable among the three arms; no mention of ALT elevations or hepatotoxicity).
- Olinger CP, Adams HP Jr, Brott TG, Biller J, Barsan WG, Toffol GJ, Eberle RW, et al. High-dose intravenous naloxone for the treatment of acute ischemic stroke. Stroke. 1990;21:721–5. [PubMed: 2339451](Among 38 patients with acute ischemic stroke given a 24 hr infusion of naloxone, side effects included nausea, vomiting, hypotension, focal seizures, and mild bilirubin elevations [peak 1.7 mg/dL], which rapidly returned to normal; no mention of ALT elevations or hepatotoxicity).
- Kaplan JL, Marx JA, Calabro JJ, Gin-Shaw SL, Spiller JD, Spivey WL, Gaddis GM, Zhao N, Harchelroad FP Jr. Double-blind, randomized study of nalmefene and naloxone in emergency department patients with suspected narcotic overdose. Ann Emerg Med. 1999;34:42–50. [PubMed: 10381993](Controlled trial of two doses of nalmefene vs naloxone in 118 patients with suspected opioid overdose found similar rates of response and no serious adverse events).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none to nalmefene, naltrexone or other agents used to treated substance abuse).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to naloxone or other opioid antagonist).
- Lucas GM, Young A, Donnell D, Richardson P, Aramrattana A, Shao Y, Ruan Y, et al. HPTN 058 study group. Hepatotoxicity in a 52-week randomized trial of short-term versus long-term treatment with buprenorphine/naloxone in HIV-negative injection opioid users in China and Thailand. Drug Alcohol Depend. 2014;142:139–45. [PMC free article: PMC4127183] [PubMed: 24999060](Among 1036 patients with opioid dependency treated with buprenorphine/naloxone short term (18 days) or long term [1 year], mean ALT levels and proportion with ALT elevations were similar in the two groups at 3, 6, 9 and 12 months).
- Soyka M, Backmund M, Schmidt P, Apelt S. Buprenorphine-naloxone treatment in opioid dependence and risk of liver enzyme elevation: results from a 12-month observational study. Am J Addict. 2014;23:563–9. [PubMed: 25251050](Among 181 patients with opioid dependency started on buprenorphine-naloxone and treated for at least 12 months, 3 to 7 had ALT elevations during visits at 3, 6, and 12 months [range 51 to 91 U/L], but all were less than 3 times ULN, and no patient developed clinically apparent liver injury or required drug discontinuation for liver test abnormalities).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to naloxone or other opioid antagonist).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to naloxone or other opioid antagonist).
- Chaignot C, Zureik M, Rey G, Dray-Spira R, Coste J, Weill A. Risk of hospitalisation and death related to baclofen for alcohol use disorders: Comparison with nalmefene, acamprosate, and naltrexone in a cohort study of 165 334 patients between 2009 and 2015 in France. Pharmacoepidemiol Drug Saf. 2018;27:1239–48. [PMC free article: PMC6282718] [PubMed: 30251424](Analysis of the French Health Insurance claims database of patients initiating alcohol use disorder therapies found an increased risk of hospitalization [+13%] and death [+31%] compared to exposure to approved drugs such as acamprosate, naltrexone and nalmefene).
- Opioids for pain. Med Lett Drugs Ther. 2018;60(1544):57–64. [PubMed: 29664446](Concise review of the efficacy, safety and costs of opioids used for pain mentions that the 3 opioid antagonists that are used to treat opioid induced constipation-methylnaltrexone, naloxegol and nalmedine-have similar degrees of efficacy and toxicities).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Opioids.[LiverTox: Clinical and Researc...]Review Opioids.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Overdoses due to fentanyl and its analogues (F/FAs) push naloxone to the limit.[J Clin Pharm Ther. 2021]Review Overdoses due to fentanyl and its analogues (F/FAs) push naloxone to the limit.Pergolizzi JV Jr, Dahan A, Ann LeQuang J, Raffa RB. J Clin Pharm Ther. 2021 Dec; 46(6):1501-1504. Epub 2021 Jun 10.
- Heroin self-administration in dependent Wistar rats: increased sensitivity to naloxone.[Psychopharmacology (Berl). 1999]Heroin self-administration in dependent Wistar rats: increased sensitivity to naloxone.Carrera MR, Schulteis G, Koob GF. Psychopharmacology (Berl). 1999 May; 144(2):111-20.
- Comparison of (+)- and (-)-Naloxone on the Acute Psychomotor-Stimulating Effects of Heroin, 6-Acetylmorphine, and Morphine in Mice.[J Pharmacol Exp Ther. 2016]Comparison of (+)- and (-)-Naloxone on the Acute Psychomotor-Stimulating Effects of Heroin, 6-Acetylmorphine, and Morphine in Mice.Eriksen GS, Andersen JM, Boix F, Bergh MS, Vindenes V, Rice KC, Huestis MA, Mørland J. J Pharmacol Exp Ther. 2016 Aug; 358(2):209-15. Epub 2016 Jun 8.
- Prescription naloxone: a novel approach to heroin overdose prevention.[Ann Emerg Med. 2007]Prescription naloxone: a novel approach to heroin overdose prevention.Sporer KA, Kral AH. Ann Emerg Med. 2007 Feb; 49(2):172-7. Epub 2006 Jul 12.
- Naloxone - LiverToxNaloxone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...