NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Nifurtimox is a nitrofuran antimicrobial agent used to treat Chagas disease (American trypanosomiasis), a chronic protozoal infection due to Trypanosoma cruzi that can lead to severe disability and death from gastrointestinal and cardiac disease. Nifurtimox is rarely associated with serum aminotransferase elevations during therapy and has not been linked to cases of clinically apparent liver injury.
Background
Nifurtimox (nye fure’ ti mox) is a nitrofuran antimicrobial agent used in the therapy of American trypanosomiasis, Chagas disease, a chronic infection with Trypanosoma cruzi. Chagas disease is a vector-borne disease transmitted by the bite of the triatomine bugs. The disease is endemic in Central and South America and cases in the United States are found predominantly in immigrants from endemic areas of the world. Chagas disease causes a mild and often asymptomatic acute infection that can be accompanied by fever, lymphadenopathy, hepatosplenomegaly, and rash. If untreated, acute infection invariably leads to a chronic infection which is asymptomatic for years or decades (indeterminate chronic infection) but progresses over years and decades to clinically apparent cardiac or gastrointestinal disease or both in up to 30% of patients. Treatment of Chagas disease focuses largely on the indeterminate chronic phase of illness with the goal of preventing the long term cardiac and gastrointestinal complications. Two medications are currently approved in the United States for Chagas disease: benznidazole and nifurtimox, and the current indications are limited to children. Nifurtimox is a prodrug that is converted to toxic radicals and DNA-damaging, reactive oxygen species by nitroreductases that are found in the mitochondria of parasites but are absent or present in low levels in humans. Nifurtimox has been shown to result in clearance of parasitemia and apparent resolution of infection in the majority of patients with Chagas disease and was approved in the United States in children from birth to age 18 years in 2021. Nifurtimox is available in tablets of 30 and 120 mg under the brand name Lampit. The currently recommended dose in children weighing 2.5 to 40 kg is 10 to 20 mg/kg per day and for those weighing 41 kg or more is 8 to 10 mg/kg per day given in 3 divided doses for 60 days. Adverse events are common and are a reason for early discontinuation of therapy in up to 10% of children. Common adverse events include anorexia, nausea and vomiting, abdominal pain, headache, dizziness, fatigue, insomnia, anxiety, rash, and neuropathy. Rare but potentially severe adverse events include embryo-fetal toxicity, hypersensitivity reactions, precipitation of acute porphyria, psychosis and carcinogenesis.
Hepatotoxicity
In multiple prospective controlled trials, nifurtimox therapy was not associated with elevations in aminotransferase or bilirubin levels or with instances of clinically apparent liver injury. Since approval of nifurtimox for American trypanosomiasis, there have been no individual reports of liver injury associated with its use.
Likelihood score: E (unlikely cause of clinically apparent liver injury when given in the recommended regimens for Chagas disease).
Mechanism of Injury
The mechanism by which nifurtimox might cause liver injury is unknown. Nifurtimox is a prodrug that is activated to an reactive metabolite by parasites but not in human cells. Nifurtimox has many diverse adverse side effects, which often limit the dose and duration of therapy and might overshadow mild degrees of liver injury due to generation of low levels of toxic metabolites in hepatocytes.
Outcome and Management
Nifurtimox therapy has not been associated with the liver injury reported. It is unknown whether there is cross sensitivity with other benzimidazoles (such as mebendazole), but there probably is and switching to another class of anthelmintic agents is appropriate if therapy is still needed.
Drug Class: Antiinfective Agents, Trypanosomiasis Agents
Other Trypanosomiasis Agents: Benznidazole, Fexinidazole
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Nifurtimox – Lampit®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
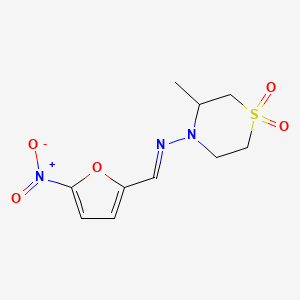
| Nifurtimox | 23256-30-6 | C10-H13-N3-O5-S |
|
ANNOTATED BIBLIOGRAPHY
References updated: 18 July 2023
Abbreviations used: NECT, nifurtimox and eflornithine combination therapy.
- Zimmerman HJ. Antihelminthics. Hepatic injury from antimicrobial agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 626-8.(Expert review of hepatotoxicity of drugs for parasite infections written in 1999, before the availability of drugs for trypanosomiasis).
- Wetzel DM, Phillips MA. Chemotherapy of protozoal infections: amebiasis, giardiasis, trichomoniasis, trypanosomiasis, Leishmaniasis, and other protozoal infections. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 987-99.(Textbook of pharmacology and therapeutics).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin American published between 1996 and 2012, none were attributed for agents used to treat trypanosomiasis including Chagas disease).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to medications for parasitic diseases).
- Forsyth CJ, Hernandez S, Olmedo W, Abuhamidah A, Traina MI, Sanchez DR, Soverow J, et al. Safety profile of nifurtimox for treatment of Chagas disease in the United States. Clin Infect Dis. 2016;63:1056-1062. [PMC free article: PMC5036918] [PubMed: 27432838](Among 53 adult U.S. immigrants from Latin America with chronic indeterminate Chagas disease treated with nifurtimox [8 to 10 mg/kg daily for 12 weeks] adverse events were frequent, mostly mild and arising during the first 2 weeks of therapy, leading to early discontinuation in 21% of patients, and including anorexia [79%], nausea [76%], headache [60%], amnesia [59%], fatigue [42%] anxiety, insomnia, and rash; no mention of ALT elevations or hepatotoxicity).
- Mesu VKBK, Kalonji WM, Bardonneau C, Mordt OV, Blesson S, Simon F, Delhomme S, et al. Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: a pivotal multicentre, randomised, non-inferiority trial. Lancet. 2018;391:144-154. [PubMed: 29113731](Among 394 patients [ages 15 years or above] with late stage African trypanosomiasis treated with fexinidazole or NECT therapy for 20 days, success rates at 18 months after enrollment were 91% vs 98% [a non-inferior result], and adverse events rates were similar [94% vs 92%] including severe adverse events [12% vs 10%]; and while ALT elevations and hepatotoxicity were not specifically mentioned, “There were no clinically significant changes in any…laboratory values over the duration of treatment”).
- Villar JC, Herrera VM, Pérez Carreño JG, Váquiro Herrera E, Castellanos Domínguez YZ, Vásquez SM, Cucunubá ZM, et al. Nifurtimox versus benznidazole or placebo for asymptomatic Trypanosoma cruzi infection (Equivalence of Usual Interventions for Trypanosomiasis - EQUITY): study protocol for a randomised controlled trial. Trials. 2019;20:431. [PMC free article: PMC6631895] [PubMed: 31307503](Protocol for a prospective randomized placebo controlled trial of benznidazole vs nifurtimox [for 60 days at full doses or 120 days at half doses] using PCR negativity on 3 occasions 12-18 months after enrollment as the primary endpoint).
- Jackson Y, Wyssa B, Chappuis F. Tolerance to nifurtimox and benznidazole in adult patients with chronic Chagas' disease. J Antimicrob Chemother. 2020;75:690-696. [PMC free article: PMC7021088] [PubMed: 31754690](Among 176 patients with chronic indeterminate Chagas disease treated with either benznidazole or nifurtimox at traditional doses for 60 days between 2008 and 2016 at a single Swiss medical center, adverse events were frequent with both regimens [90%], commonly pruritus [37% vs 20%], rash [29% vs 14%], anorexia [22% vs 74%], nausea [36% vs 55%], abdominal pain [22% vs 39%], headache [40% vs 71%], neuropathy [21% vs 5%], vertigo [7% vs 27%], insomnia [26% vs 50%], psychosis [1% vs 1%], fatigue [44% vs 69%], fever [0% vs 14%], arthralgia [4% vs 26%], with only 63% of patients completing therapy; no mention of ALT elevations or hepatotoxicity).
- Berenstein AJ, Falk N, Moscatelli G, Moroni S, González N, Garcia-Bournissen F, Ballering G, et al. Adverse events associated with nifurtimox treatment for Chagas disease in children and adults. Antimicrob Agents Chemother. 2021;65:e01135-20. [PMC free article: PMC7849004] [PubMed: 33168612](Among 320 patients with Chagas disease [215 children, 105 adults] treated with nifurtimox in a single Argentinian medical center between 1980 and 2019, adverse events were common [40%], were more frequent in adults than children [61% vs 29%] and included anorexia, weight loss, irritability, headache, nausea, vomiting, and rash leading to early discontinuation in 10% of patients; no mention of ALT elevations or hepatotoxicity).
- Altcheh J, Castro L, Dib JC, Grossmann U, Huang E, Moscatelli G, Pinto Rocha JJ, et al.; CHICO Study Group. Prospective, historically controlled study to evaluate the efficacy and safety of a new paediatric formulation of nifurtimox in children aged 0 to 17 years with Chagas disease one year after treatment (CHICO). PLoS Negl Trop Dis. 2021;15:e0008912. [PMC free article: PMC7790535] [PubMed: 33412557](Among 330 children with Chagas disease treated with nifurtimox for 30 or 60 days, serological responses were more frequent with longer therapy [33% vs 19%], while PCR positivity fell from 52% initially to 1% at one year in both groups; adverse event rates were similar [28% vs 26%]; no mention of ALT elevations or hepatotoxicity).
- Falk N, Berenstein AJ, Moscatelli G, Moroni S, González N, Ballering G, Freilij H, et al. Effectiveness of nifurtimox in the treatment of Chagas disease: a long-term retrospective cohort study in children and adults. Antimicrob Agents Chemother. 2022;66:e0202121. [PMC free article: PMC9112880] [PubMed: 35416710](Among 289 patients with Chagas disease treated with nifurtimox at a single medical center in Argentina, all except one cleared parasitemia by the end of treatment, 35% had seroconversion and 58% seroreduction).
- Abbott A, Montgomery SP, Chancey RJ. Characteristics and adverse events of patients for whom nifurtimox was released through CDC-sponsored investigational new drug program for treatment of Chagas disease - United States, 2001-2021. MMWR Morb Mortal Wkly Rep. 2022;71:371-374. [PMC free article: PMC8911997] [PubMed: 35271563](Among 336 patients with Chagas disease who were treated with nifurtimox under a CDC approved Investigation New Drug treatment protocol between 2001-2021, ages ranged from 1 to 78 years, 93% were Hispanic, and 91% had at least one adverse event, 21% being reported as severe, most commonly nausea, anorexia, weight loss, headache, and abdominal pain, but only one case of elevated ALT levels and no instance of severe hepatic adverse events).
- Altcheh J, Sgierra V, Ramirez T, Pinto Rocha JJ, Grossmann U, Huang E, Moscatelli G, et al. Efficacy and Safety of Nifurtimox in Pediatric Patients with Chagas Disease: Results at 4-Year Follow-Up in a Prospective, Historically Controlled Study (CHICO SECURE). Antimicrob Agents Chemother. 2023;67:e0119322. [PMC free article: PMC10112190] [PubMed: 36975790](Four year follow up on 295 children treated with nifurtimox in the CHICO study [Altcheh 2021], all of whom remained free of signs or symptoms of cardiomyopathy and more than 90% remained T cruzi PCR negative while there were no new adverse events).
- Hochberg NS, Montgomery SP. Chagas Disease. Ann Intern Med. 2023;176:ITC17-ITC32. [PMC free article: PMC10442057] [PubMed: 36780647](Review of the risk factors and causes, clinical features and natural history, epidemiology, pathogenesis, diagnosis, management and treatment of American trypanosomiasis known as Chagas disease after the scientist who first identified the causative parasite).
- Torrico F, Gascón J, Ortiz L, Pinto J, Rojas G, Palacios A, Barreira F, et al. A phase 2, randomized, multicenter, placebo-controlled, proof-of-concept trial of oral fexinidazole in adults with chronic indeterminate Chagas Disease. Clin Infect Dis. 2023;76:e1186-e1194. [PMC free article: PMC9907522] [PubMed: 35925555](Among 47 adults with chronic indeterminate Chagas disease treated with 1 of 6 regimens of fexinidazole given orally for 2, 4 or 8 weeks or placebo [n=7], rapid sustained clearance of parasitemia was achieved in all subjects but adverse events included delayed onset neutropenia [20%] and serum aminotransferase elevations [40% above 3 times ULN], which often arose after therapy was stopped and persisted beyond 3 months in 23% but were asymptomatic and ultimately resolved in all).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Update on nifurtimox for treatment of Chagas disease.[Drugs Today (Barc). 2021]Review Update on nifurtimox for treatment of Chagas disease.Thakare R, Dasgupta A, Chopra S. Drugs Today (Barc). 2021 Apr; 57(4):251-263.
- Nifurtimox versus benznidazole or placebo for asymptomatic Trypanosoma cruzi infection (Equivalence of Usual Interventions for Trypanosomiasis - EQUITY): study protocol for a randomised controlled trial.[Trials. 2019]Nifurtimox versus benznidazole or placebo for asymptomatic Trypanosoma cruzi infection (Equivalence of Usual Interventions for Trypanosomiasis - EQUITY): study protocol for a randomised controlled trial.Villar JC, Herrera VM, Pérez Carreño JG, Váquiro Herrera E, Castellanos Domínguez YZ, Vásquez SM, Cucunubá ZM, Prado NG, Hernández Y. Trials. 2019 Jul 15; 20(1):431. Epub 2019 Jul 15.
- Review Chagas disease: progress and new perspectives.[Curr Med Chem. 2010]Review Chagas disease: progress and new perspectives.Sánchez-Sancho F, Campillo NE, Páez JA. Curr Med Chem. 2010; 17(5):423-52.
- Review American trypanosomiasis (Chagas disease).[Infect Dis Clin North Am. 2012]Review American trypanosomiasis (Chagas disease).Rassi A Jr, Rassi A, Marcondes de Rezende J. Infect Dis Clin North Am. 2012 Jun; 26(2):275-91.
- Trypanocidal drugs for late-stage, symptomatic Chagas disease (Trypanosoma cruzi infection).[Cochrane Database Syst Rev. 2020]Trypanocidal drugs for late-stage, symptomatic Chagas disease (Trypanosoma cruzi infection).Vallejo M, Reyes PP, Martinez Garcia M, Gonzalez Garay AG. Cochrane Database Syst Rev. 2020 Dec 11; 12(12):CD004102. Epub 2020 Dec 11.
- Nifurtimox - LiverToxNifurtimox - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...