NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Nystatin is a topical and oral antifungal agent with activity against many species of yeast and candida albicans, which is used largely to treat skin and oropharyngeal candidiasis. Nystatin is not absorbed orally and has not been linked to drug induced liver injury.
Background
Nystatin (nye stat' in) is a polyene macrolide antibiotic that acts by binding to sterols in the plasma membranes of fungi causing the cells to leak, eventually leading to fungal cell death. Nystatin is indicated for the treatment of candidal infections of the skin, mucous membranes and gastrointestinal tract. It is not absorbed orally and thus not indicated for invasive fungal infections. Nystatin was approved by the FDA in 1971 and is currently widely used in the treatment of superficial candida infections of the skin, mucous membranes and gastrointestinal tract, including oropharyngeal candidiasis. Nystatin is available in multiple forms such as tablets, troches, powder for suspension, creams and ointments and varying concentrations which are usually measured in units. Nystatin is available in generic forms and under brand names such as Mycostatin, Nilstat, Nystat and Nystop. The recommended dose for oropharyngeal candidiasis is 500,000 to 1,000,000 units 3 to 5 times daily as oral suspension or tablets (dissolved in the mouth) for 1 to 2 weeks. Common side effects include metallic taste, dry mouth, anorexia and nausea.
Hepatotoxicity
Nystatin therapy has been associated with a low rate of serum enzyme abnormalities, although it has been difficult to attribute these elevations to nystatin. Despite its use for several decades, there have been no convincing cases of acute hepatic injury linked to nystatin therapy. While nystatin is usually is not normally absorbed, low concentrations may enter the circulation in patients with inflammation and damage to the gastrointestinal tract. Nevertheless, nystatin is considered very safe and is unlikely to cause hepatic injury.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The absence of hepatotoxicity from nystatin is probably largely due to lack of absorption.
Drug Class: Antifungal Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Nystatin – Generic, Mycostatin®, Nystat®
DRUG CLASS
Antifungal Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Nystatin | 1400-61-9 | C46-H83-N-O18 C47-H75-N-O17 |
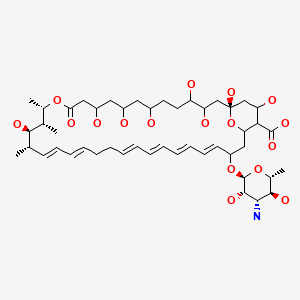
|
ANNOTATED BIBLIOGRAPHY
References updated: 30 April 2020
- Zimmerman HJ. Antifungal agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 609-11.(Expert review of hepatotoxicity of antifungal agents published in 1999; nystatin is considered safe because it is not absorbed).
- Moseley RH. Antifungal agents. Antibacterial and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 470-81.(Review of hepatotoxicity of antifungal agents does not discuss nystatin).
- Rogers PD, Krysan DJ. Antifungal agents. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1087-104.(Textbook of pharmacology and therapeutics).
- Pons V, Greenspan D, Lozada-Nur F, McPhail L, Gallant JE, Tunkel A, Johnson CC, et al. Oropharyngeal candidiasis in patients with AIDS: randomized comparison of fluconazole versus nystatin oral suspensions. Clin Infect Dis. 1997;24:1204–7. [PubMed: 9195083](Controlled trial of 2 weeks of nystatin vs fluconazole in 167 patients with HIV infection and oropharyngeal candidiasis; side effects were minimal and included liver enzyme elevations in 2 subjects on fluconazole, but none on nystatin).
- Pons V, Greenspan D, Debruin M. Therapy for oropharyngeal candidiasis in HIV-infected patients: a randomized, prospective multicenter study of oral fluconazole versus clotrimazole troches. The Multicenter Study Group. J Acquir Immune Defic Syndr. 1993;6:1311–6. [PubMed: 8254467](Trial of 14 days of fluconazole vs clotrimazole in 334 HIV infected patients with oropharyngeal candidiasis; 2 patients on fluconazole but none of clotrimazole were withdrawn for serum ALT elevations).
- Flynn PM, Cunningham CK, Kerkering T, San Jorge AR, Peters VB, Pitel PA, Harris J, et al. Oropharyngeal candidiasis in immunocompromised children: a randomized, multicenter study of orally administered fluconazole suspension versus nystatin. The Multicenter Fluconazole Study Group. J Pediatr. 1995;127:322–8. [PubMed: 7636666](Controlled trial of 14 days of fluconazole vs nystatin in 182 children with oropharyngeal candidiasis; liver test abnormalities occurred in 8% on nystatin vs 7% on fluconazole; no early discontinuations because of abnormalities).
- Young GA, Bosly A, Gibbs DL, Durrant S. A double-blind comparison of fluconazole and nystatin in the prevention of candidiasis in patients with leukaemia. Antifungal Prophylaxis Study Group. Eur J Cancer. 1999;35:1208–13. [PubMed: 10615231](Controlled trial of nystatin vs fluconazole for prevention of fungal infections in 160 patients with leukemia on chemotherapy; ALT elevations occurred in 24% of nystatin vs 24% of fluconazole treated patients, but most were attributed to other causes; one patient in each group developed “hepatitis”).
- Drugs for vulvovaginal candidiasis. Med Lett Drugs Ther. 2001;43(1095):3–4. [PubMed: 11151090](Concise summary of agents approved for therapy of vulvovaginal candidiasis including topical nystatin and the “azoles” such as clotrimazole, miconazole and butoconazole and oral fluconazole, mentions that nystatin is less effective than the azoles and that a single oral dose of fluconazole is often preferred by patients).
- Antifungal drugs. Treat Guidel Med Lett. 2009;7:95–102. [PubMed: 19940816](Concise summary of therapy of fungal infections with recommendations on agents, dosage and duration of treatment and safety; nystatin is listed as a topical antifungal agent, but is not discussed).
- Pienaar ED, Young T, Holmes H. Interventions for the prevention and management of oropharyngeal candidiasis associated with HIV infection in adults and children. Cochrane Database Syst Rev. 2010;11:CD003940. [PMC free article: PMC7156835] [PubMed: 21069679](Metaanalysis of trials comparing different regimens of therapy for oropharyngeal candidiasis; does not discuss hepatotoxicity).
- Antifungal drugs. Treat Guidel Med Lett. 2012;10(120):61–8. [PubMed: 22825657](Concise summary of therapy of fungal infections with recommendations on agents, dosage and duration of treatment and safety; nystatin is listed as a topical antifungal agent, but is not discussed).
- Pankhurst CL. Candidiasis (oropharyngeal). BMJ Clin Evid. 2013;2013:1304. [PMC free article: PMC3821534] [PubMed: 24209593](Review of the evidence for efficacy of pharmacologic prevention and treatment or oropharyngeal candidiasis concludes that nystatin is less effective than oral and topical azole therapies but adverse events are uncommon; no mention of hepatotoxicity of nystatin).
- Mersal A, Alzahrani I, Azzouz M, Alsubhi A, Alsawaigh H, Albshri N, Bajammal M, et al. Oral nystatin versus intravenous fluconazole as neonatal antifungal prophylaxis: non-inferiority trial. J Clin Neonatol. 2013;2:88–92. [PMC free article: PMC3775143] [PubMed: 24049751](Among 57 premature neonates treated with oral nystatin or intravenous fluconazole [every 48-72 hours] for 6 weeks, none developed invasive candida infections and detection of candida in rectal swabs was uncommon in both groups; serum ALT levels were higher in those who received fluconazole but were all in the normal range).
- Fan S, Liu X, Wu C, Xu L, Li J. Vaginal nystatin versus oral fluconazole for the treatment for recurrent vulvovaginal candidiasis. Mycopathologia. 2015;179(1-2):95–101. [PubMed: 25416649](Among 293 women with recurrent vulvovaginal candidiasis treated with vaginal nystatin suppositories for 14 days monthly or oral fluconazole [150 mg] once weekly, initial and 6 month follow up cure rates were similar in the two groups, and only one patient was intolerant of vaginal nystatin [burning and pain] and required switching to fluconazole).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 14 [1.6%] were attributed to an antifungal agent, but none to nystatin).
- Lyu X, Zhao C, Yan ZM, Hua H. Efficacy of nystatin for the treatment of oral candidiasis: a systematic review and meta-analysis. Drug Des Devel Ther. 2016;10:1161–71. [PMC free article: PMC4801147] [PubMed: 27042008](Systematic review of the published literature on the efficacy and safety of nystatin therapy for oral candidiasis identified 11 trials in 1148 patients [dental, pediatric, HIV-AIDS, cancer and miscellaneous groups], with cure rates of 14-77%; adverse events being reported in 8 studies common mentioned change in taste, and less commonly nausea, vomiting, diarrhea, anorexia and abdominal pain; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- In vitro susceptibility profile of 200 recent clinical isolates of Candida spp. to topical antifungal treatments of vulvovaginal candidiasis, the imidazoles and nystatin agents.[J Mycol Med. 2014]In vitro susceptibility profile of 200 recent clinical isolates of Candida spp. to topical antifungal treatments of vulvovaginal candidiasis, the imidazoles and nystatin agents.Choukri F, Benderdouche M, Sednaoui P. J Mycol Med. 2014 Dec; 24(4):303-7. Epub 2014 Oct 23.
- An ex-vivo oral mucosa infection model for the evaluation of the topical activity of antifungal agents.[Mycoses. 2008]An ex-vivo oral mucosa infection model for the evaluation of the topical activity of antifungal agents.Ohnemus U, Willers C, Bubenheim M, Horstkotte MA, Houdek P, Fischer F, Schmage P, Moll I, Brandner JM. Mycoses. 2008 Jan; 51(1):21-9.
- Synergistic effect of thymoquinone and nystatin in the treatment of oral candidiasis; an in vitro study.[Odontology. 2022]Synergistic effect of thymoquinone and nystatin in the treatment of oral candidiasis; an in vitro study.Özdal Zincir Ö, Özdal U, Ünlü Ö, Demirci M, Katiboğlu AB, Egil E, Altan Şallı G. Odontology. 2022 Apr; 110(2):330-337. Epub 2021 Oct 17.
- Review Prophylactic oral/topical non-absorbed antifungal agents to prevent invasive fungal infection in very low birth weight infants.[Cochrane Database Syst Rev. 2009]Review Prophylactic oral/topical non-absorbed antifungal agents to prevent invasive fungal infection in very low birth weight infants.Austin N, Darlow BA, McGuire W. Cochrane Database Syst Rev. 2009 Oct 7; (4):CD003478. Epub 2009 Oct 7.
- Review A reevaluation of nystatin in prophylaxis and treatment of oropharyngeal candidiasis.[Rev Esp Quimioter. 1998]Review A reevaluation of nystatin in prophylaxis and treatment of oropharyngeal candidiasis.Alvarez Alvarez ME, Sánchez-Sousa A, Baquero F. Rev Esp Quimioter. 1998 Dec; 11(4):295-315.
- Nystatin - LiverToxNystatin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...