NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Paliperidone is a second generation (atypical) antipsychotic agent that is available in both oral and long acting parenteral forms and is used in the treatment of schizophrenia and schizoaffective disorder. Paliperidone is associated with a low rate of serum aminotransferase elevations during therapy, but has not been linked to instances of clinically apparent acute liver injury.
Background
Paliperidone (pal" ee per' i done) is a second generation antipsychotic agent which appears to act as a dopamine type 2 (D2) partial agonist and post-synaptic antagonist and serotonin (5-HT)-2A receptor antagonist and is similar in structure and mechanism of action to risperidone. Indeed, paliperidone is the primary active metabolite of risperidone, its chemical name being 9-hydroxyrisperidone. Several randomized controlled trials have shown that oral paliperidone improves symptoms of schizophrenia and is comparable in effect to risperidone and ziprasidone. Oral formulations of paliperidone were approved for use in the United States in 2006 as treatment for schizophrenia and schizoaffective disorder, as extended release tablets of 1.5, 3, 6 and 9 mg generically and under the brand name Invega. The recommended maintenance dose is 3 to 12 mg daily, and it is approved for use in adolescents (ages 12 to 17 years) as well as adults. Subsequently, parenteral formulations of paliperidone palmitate were developed that could be administered every one or three months. These palmitate formulations are given intramuscularly in varying doses and are available under the brand names Invega Sustenna and Invega Trinzia. Common side effects of paliperidone include dizziness, dry mouth, somnolence, fatigue, nasal congestion, anxiety, restlessness and weight gain. Paliperidone therapy is also associated with postural hypotension and prolongation of the QTc interval. The intramuscular formulations also can cause local injection site and hypersensitivity reactions. Rare, but potential severe adverse reactions (mentioned in most antipsychotic and antidepressant product labels) include excess mortality in elderly patients with dementia, tardive dyskinesia, major neurologic events, neuroleptic malignant syndrome, thrombotic thrombocytopenic purpura, orthostatic hypotension, prolongation of the QT interval, dyslipidemia, hyperglycemia, seizures and neutropenia.
Hepatotoxicity
Liver test abnormalities occur in up to 1% of patients receiving paliperidone, but similar rates have been reported with placebo therapy and with comparator agents. The ALT elevations are usually mild, transient and often resolve even without dose modification or drug discontinuation. There have been no published reports of clinically apparent liver injury with symptoms or jaundice attributed solely to paliperidone therapy, even with the long acting parenteral formulations.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which paliperidone might cause serum ALT elevations or liver injury is not known. Paliperidone is metabolized to some extent by the cytochrome P450 system (CYP 2D6 and 3A4), but is an uncommon cause of significant drug-drug interactions with agents that inhibit or induce these microsomal enzymes.
Outcome and Management
The serum aminotransferase elevations that occur on paliperidone therapy are usually self-limited and often do not require dose modification or discontinuation. No instances of acute liver failure, chronic hepatitis or vanishing bile duct syndrome have been attributed to paliperidone. Cross sensitivity to liver related or other hypersensitivity reactions between paliperidone and structurally related antipsychotic agents (such as iloperidone, lurasidone, risperidone and ziprasidone) have not been demonstrated, but may well occur.
Drug Class: Antipsychotic Agents, Atypicals
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Paliperidone – Generic, Invega®
DRUG CLASS
Antipsychotic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
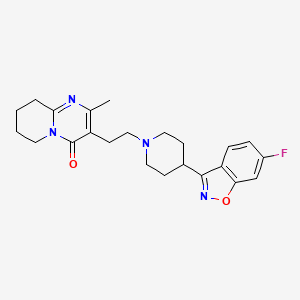
| Paliperidone | 144598-75-4 | C23-H27-F-N4-O3 |

|
| Risperidone | 106266-06-2 | C23-H27-F-N4-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 06 June 2023
- Meyer JM. Pharmacotherapy of psychosis and mania. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 279-302.(Textbook of pharmacology and therapeutics).
- Larry D. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 2nd ed. New York: Informa Healthcare USA, 2007, pp. 507-26.(Review of hepatotoxicity of psychiatric agents does not discuss paliperidone).
- Canuso CM, Lindenmayer JP, Kosik-Gonzalez C, Turkoz I, Carothers J, Bossie CA, Schooler NR. A randomized, double-blind, placebo-controlled study of 2 dose ranges of paliperidone extended-release in the treatment of subjects with schizoaffective disorder. J Clin Psychiatry. 2010;71:587–98. [PubMed: 20492853](Among 316 patients with schizoaffective disorder treated with paliperidone [6 or 12 mg daily] or placebo for 6 weeks, symptom scores improved more with the higher dose paliperidone; adverse events included headache, tremor, somnolence and prolactin elevations, and one patient on paliperidone had “a markedly abnormal elevation” in ALT; no further details provided).
- Pandina GJ, Lindenmayer JP, Lull J, Lim P, Gopal S, Herben V, Kusumakar V, et al. A randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. J Clin Psychopharmacol. 2010;30:235–44. [PubMed: 20473057](Among 652 patients with an acute exacerbation of schizophrenia treated with monthly injections of paliperidone [25, 100 or 150 mg equivalents] or placebo for 13 weeks, symptom scores improved more with paliperidone than placebo and “there were no clinically relevant changes from baseline in… clinical laboratory parameters”).
- Gopal S, Hough DW, Xu H, Lull JM, Gassmann-Mayer C, Remmerie BM, Eerdekens MH, et al. Efficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response study. Int Clin Psychopharmacol. 2010;25:247–56. [PubMed: 20389255](Among 388 patients with schizophrenia treated with paliperidone palmitate [50, 100 or 150 mg eq] or placebo by intramuscular injection once monthly, symptom scores improved with the active drug and adverse events included headache, vomiting, pain and injection site reactions; no mention of ALT elevations or hepatotoxicity).
- Kramer M, Litman R, Hough D, Lane R, Lim P, Liu Y, Eerdekens M. Paliperidone palmitate, a potential long-acting treatment for patients with schizophrenia. Results of a randomized, double-blind, placebo-controlled efficacy and safety study. Int J Neuropsychopharmacol. 2010;13:635–47. [PubMed: 19941696](Among 247 patients with schizophrenia treated with intramuscular paliperidone palmitate or placebo, one patient in the placebo group developed “elevated hepatic enzymes”, but no serious adverse events were liver related).
- Emsley R, Berwaerts J, Eerdekens M, Kramer M, Lane R, Lim P, Hough D, Palumbo J. Efficacy and safety of oral paliperidone extended-release tablets in the treatment of acute schizophrenia: pooled data from three 52-week open-label studies. Int Clin Psychopharmacol. 2008;23:343–56. [PubMed: 18854723](Among 1083 patients with schizophrenia treated with open label extension studies of paliperidone for 52 weeks, common side effects included insomnia, headache and restlessness [akathisia] and “there were no clinically meaningful changes” in clinical chemistry results).
- Meltzer HY, Bobo WV, Nuamah IF, Lane R, Hough D, Kramer M, Eerdekens M. Efficacy and tolerability of oral paliperidone extended-release tablets in the treatment of acute schizophrenia: pooled data from three 6-week, placebo-controlled studies. J Clin Psychiatry. 2008;69:817–29. [PubMed: 18466043](Among 1325 patients with acute schizophrenia treated with paliperidone [3-15 mg daily], olanzapine or placebo for 6 weeks in 3 controlled trials, adverse events were more common with the higher doses; no mention of ALT elevations or hepatotoxicity and no liver related serious adverse events reported).
- Parsons B, Allison DB, Loebel A, Williams K, Giller E, Romano S, Siu C. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr Res. 2009;110:103–10. [PubMed: 19321312](Analysis of weight gain in 21 placebo controlled trials [~3300 patients]; average monthly weight gain in pounds was +0.1 with placebo, +0.8 olanzapine, 0.6 risperidone, -0.3 ziprasidone; a 5% increase in weight occurred after one year in 13% of placebo, 39% haloperidol, 20% ziprasidone, 45% risperidone and 60% olanzapine treated subjects; no mention of paliperidone).
- Coppola D, Liu Y, Gopal S, Remmerie B, Samtani MN, Hough DW, Nuamah I, Sulaiman A, Pandina G. A one-year prospective study of the safety, tolerability and pharmacokinetics of the highest available dose of paliperidone palmitate in patients with schizophrenia. BMC Psychiatry. 2012;12:26. [PMC free article: PMC3384238] [PubMed: 22455454](Among 212 patients with schizophrenia treated with different doses of monthly intramuscular injections of paliperidone palmitate, adverse events included local injection site reactions, nasopharyngitis, insomnia, headache, tachycardia, akathisia, tremor and weight gain; no mention of ALT elevations or hepatotoxicity).
- Berwaerts J, Melkote R, Nuamah I, Lim P. A randomized, placebo- and active-controlled study of paliperidone extended-release as maintenance treatment in patients with bipolar I disorder after an acute manic or mixed episode. J Affect Disord. 2012;138:247–58. [PubMed: 22377512](Among 756 patients with mania or mixed episodes treated with paliperidone [3-12 mg daily] or olanzapine or placebo, adverse events were most frequent with olanzapine; no mention of ALT elevations or hepatotoxicity).
- Citrome L. Oral paliperidone extended-release: chemistry, pharmacodynamics, pharmakinetics and metabolism, clinical efficacy, safety and tolerability. Expert Opin Drug Metab Toxicol. 2012;8:873–88. [PubMed: 22632481](Review of the structure, mechanism of action, clinical efficacy and safety of paliperidone as therapy of schizophrenia mentions that adverse events include extrapyramidal symptoms, restlessness, tachycardia, headache, anxiety, somnolence and weight gain, as well as prolongation of the QTc interval; no mention of ALT elevations or hepatotoxicity).
- Drugs for psychiatric disorders. Treat Guidel Med Lett. 2013;11(130):53–64. [PubMed: 23715100](Concise review of safety, efficacy and role of drugs for psychiatric disorders mentions that paliperidone is a second generation antipsychotic agent whose adverse side effects include extrapyramidal symptoms, prolactin elevation, nausea, somnolence, dizziness, tachycardia and QTc interval prolongation; no mention of ALT elevations or hepatotoxicity).
- Alphs L, Mao L, Rodriguez SC, Hulihan J, Starr HL. Design and rationale of the Paliperidone Palmitate Research in Demonstrating Effectiveness (PRIDE) study: a novel comparative trial of once-monthly paliperidone palmitate versus daily oral antipsychotic treatment for delaying time to treatment failure in persons with schizophrenia. J Clin Psychiatry. 2014;75:1388–93. [PubMed: 25375367](Description of 15 month trial design to compare one monthly injections of paliperidone to daily oral administration, the end points being time to relapse and safety).
- Musil R, Obermeier M, Russ P, Hamerle M. Weight gain and antipsychotics: a drug safety review. Expert Opin Drug Saf. 2015;14:73–96. [PubMed: 25400109](Extensive systematic review of the literature on the problem of weight gain during therapy with antipsychotic agents, mentions that weight gain of 7% or more occurs in 0-29% of patients on paliperidone averaging 1.2 kg, the rates being lower than with olanzapine, but higher than with aripiprazole).
- Ravenstijn P, Remmerie B, Savitz A, Samtani MN, Nuamah I, Chang CT, De Meulder M, et al. Pharmacokinetics, safety, and tolerability of paliperidone palmitate 3-month formulation in patients with schizophrenia: A phase-1, single-dose, randomized, open-label study. J Clin Pharmacol. 2016;56:330–9. [PubMed: 26189570](Among 328 patients with schizophrenia treated with varying doses of a parenteral formulation of paliperidone given every 3 months, common side effects were headache, weight gain and anxiety, but there were “no clinically relevant changes” in chemistry results).
- Berwaerts J, Liu Y, Gopal S, Nuamah I, Xu H, Savitz A, Coppola D, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72:830–9. [PubMed: 25820612](Among 506 patients with schizophrenia treated with a 1- and 3-month formulations of paliperidone or placebo, the most common adverse events were headache, weight gain, nasopharyngitis and restlessness [akathisia: 4%]; the only treatment related early discontinuation was for ALT elevations in a placebo recipient).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 patients with drug induced liver injury seen over a ten year period at 8 US medical centers, one case was attributed to olanzapine, but none to paliperidone or other atypical antipsychotic medications).
- Drugs for psychotic disorders. Med Lett Drugs Ther. 2016;58(1510):160–4. [PubMed: 27960194](Concise review of medications available in the US for therapy of psychotic disorders; mentions that olanzapine can cause aminotransferase elevations, and that olanzapine and ziprasidone can cause DRESS syndrome, but does not mention ALT elevations or hepatotoxicity for any of agents discussed, including aripiprazole, brexpiprazole, cariprazine, clozapine, quetiapine, risperidone, asenapine, iloperidone, paliperidone and lurasidone).
- Chang CH, Lane HY, Liu CY, Chen SJ, Lin CH. Paliperidone is associated with reduced risk of severe hepatic outcome in patients with schizophrenia and viral hepatitis: A nationwide population-based cohort study. Psychiatry Res. 2019;281:112597. [PubMed: 31629300](Analysis of a national health care database found that a cohort of 134 patients with viral hepatitis and schizophrenia who were receiving paliperidone compared to 268 controls matched for age, sex and year enrolled who were receiving other atypical antipsychotics had a lower rate of severe hepatic outcomes [decompensation, liver failure, transplantation or cancer] when matched for age, sex and year enrolled).
- Castanheira L, Fernandes E, Levy P, Coentre R. Aripiprazole-induced hepatitis: a case report. Clin Psychopharmacol Neurosci. 2019;17:551–555. [PMC free article: PMC6852676] [PubMed: 31671495](28 year old black woman developed jaundice 21 days after starting aripiprazole [bilirubin 1.1 total, 0.8 mg/dL direct, ALT 745 U/L, Alk P 224 U/L], with resolution within 3 weeks of switching to paliperidone).
- Khorassani F, Sousonis F, Lopez LV. Risperidone- and paliperidone-induced hepatotoxicity: Case report and review of literature. Am J Health Syst Pharm. 2020;77:1578–1584. [PubMed: 32699878](23 year old man with schizophrenia developed rising ALT when started on risperidone [ALT 25 to 489 U/L] with no elevations in alkaline phosphatase or AST, which decreased when switched to paliperidone but did not become normal until switched to haloperidol).
- Druschky K, Toto S, Bleich S, Baumgärtner J, Engel RR, Grohmann R, Maier HB, et al. Severe drug-induced liver injury in patients under treatment with antipsychotic drugs: data from the AMSP study. World J Biol Psychiatry. 2021;22:373–386. [PubMed: 32892689](Among 246 cases of severe liver injury due to antipsychotic medications identified in a prospective registry of German psychiatric hospitals between 1993 and 2016, 46 arose in 38,349 patients [0.12%] who received clozapine [34 as a single antipsychotic agent]; other commonly implicated agents being olanzapine [n=90 of 54,822: 0.16%], quetiapine [34 of 66,209: 0.05%] and risperidone [27 of 51,683: 0.05%]; two fatal cases occurred in olanzapine-treated patients; low rates were found for ziprasidone [no cases among 3568 patients treated] and aripiprazole [6 cases of 15,988 patients treated: 0.01%] paliperidone is not mentioned).
- Zeiss R, Hafner S, Schönfeldt-Lecuona C, Connemann BJ, Gahr M. Drug-associated liver injury related to antipsychotics: exploratory analysis of pharmacovigilance data. J Clin Psychopharmacol. 2022;42:440–444. [PubMed: 35730552](Review of the VigiBase data base of individual case safety reports on antipsychotics and liver injury found positive hepatic safety signals for olanzapine and clozapine, but not for paliperidone, risperidone, quetiapine, ziprasidone, asenapine, aripiprazole, brexpiprazole, and cariprazine).
- Alphs L, Brown B, Turkoz I, Baker P, Fu DJ, Nuechterlein KH. The Disease Recovery Evaluation and Modification (DREaM) study: Effectiveness of paliperidone palmitate versus oral antipsychotics in patients with recent-onset schizophrenia or schizophreniform disorder. Schizophr Res. 2022;243:86–97. [PubMed: 35247794](Among 234 adults with recent onset schizophrenia or schizoaffective disorder on oral antipsychotic agents who were either continued on the oral agent or switched to one or three monthly injections of paliperidone for 9 months, the time to relapse was identical in all groups, but became lower with the parenteral drug when patients were then re-randomized; adverse event rates were similar in the two groups except for weight gain which was greater with paliperidone injections [4.8 vs 2.7 kg at 9 months]; no mention of ALT elevations or hepatotoxicity).
- Gunther M, Dopheide JA. Antipsychotic safety in liver disease: a narrative review and practical guide for the clinician. J Acad Consult Liaison Psychiatry. 2023;64:73–82. [PubMed: 36180017](Review of the literature on hepatotoxicity of antipsychotic medications and guidance on their use in patients with liver disease characterizes chlorpromazine, clozapine, and olanzapine as having the greatest risk for causing liver injury, quetiapine and risperidone as having moderate risk, haloperidol as having low risk, and paliperidone, aripiprazole, lurasidone, loxapine, and ziprasidone as having the lowest risk).
- Cicala G, de Filippis R, Barbieri MA, Cutroneo PM, De Fazio P, Schoretsanitis G, Spina E. Tolerability profile of paliperidone palmitate formulations: A pharmacovigilance analysis of the EUDRAVigilance database. Front Psychiatry. 2023;14:1130636. [PMC free article: PMC10116827] [PubMed: 37091708](Analysis of spontaneous adverse event reports related to second generation long acting injectable antipsychotic agents to the European Union Drug Regulating Authorities Vigilance registry between 2011 and 2020 identified 8152 reports attributed to paliperidone, 5,317 risperidone, 4038 aripiprazole, 2802 olanzapine, there was a disproportional rate of sexual and fertility disorders and edema with paliperidone vs other agents, but similar rates of extrapyramidal symptoms and “hepatobiliary disorders” [0.9% vs 1.0% of reports]).
- Giron-Hernandez C, Han JH, Alberio R, Singh A, García-Portilla MP, Pompili M, Knight RK, et al. Efficacy and safety of paliperidone palmitate 6-month versus paliperidone palmitate 3-month long-acting injectable in European patients with schizophrenia: a post hoc analysis of a global phase-3 double-blind randomized non-inferiority study. Neuropsychiatr Dis Treat. 2023;19:895–906. [PMC free article: PMC10108905] [PubMed: 37077705](Among 384 patients with schizophrenia stabilized on injectable paliperidone who were treated with either 3-monthly [350 or 525 mg] or 6-monthly [700 or 1000 mg] injections, the 12 month relapse rate was higher but not inferior with the 6-monthly dosing [6.9% vs 2.4%] and adverse event rates were similar in the two groups: no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial.[JAMA. 2014]Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial.McEvoy JP, Byerly M, Hamer RM, Dominik R, Swartz MS, Rosenheck RA, Ray N, Lamberti JS, Buckley PF, Wilkins TM, et al. JAMA. 2014 May 21; 311(19):1978-87.
- Letter to the Editor: Rethinking The Cost Of Antipsychotic Treatment: The Average Cost Of The Drugs Used In Turkey In 2020.[Turk Psikiyatri Derg. 2022]Letter to the Editor: Rethinking The Cost Of Antipsychotic Treatment: The Average Cost Of The Drugs Used In Turkey In 2020.Yıldız M, Osman E. Turk Psikiyatri Derg. 2022 Summer; 33(2):146-148.
- Review A critical appraisal of paliperidone long-acting injection in the treatment of schizoaffective disorder.[Ther Clin Risk Manag. 2016]Review A critical appraisal of paliperidone long-acting injection in the treatment of schizoaffective disorder.Chue P, Chue J. Ther Clin Risk Manag. 2016; 12:109-16. Epub 2016 Jan 27.
- Review Paliperidone to Treat Psychotic Disorders.[Neurol Int. 2021]Review Paliperidone to Treat Psychotic Disorders.Minwalla HD, Wrzesinski P, Desforges A, Caskey J, Wagner B, Ingraffia P, Patterson JC 2nd, Edinoff AN, Kaye AM, Kaye AD, et al. Neurol Int. 2021 Jul 28; 13(3):343-358. Epub 2021 Jul 28.
- Review Pharmacokinetic drug evaluation of paliperidone in the treatment of schizoaffective disorder.[Expert Opin Drug Metab Toxicol...]Review Pharmacokinetic drug evaluation of paliperidone in the treatment of schizoaffective disorder.Macaluso M, Oliver H, Sohail Z. Expert Opin Drug Metab Toxicol. 2017 Aug; 13(8):871-879. Epub 2017 Jul 12.
- Paliperidone - LiverToxPaliperidone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...