NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Paxlovid is a co-packaged combination of nirmatrelvir, a second generation protease inhibitor, and ritonavir, a pharmacological enhancer, that is used to treated infection with the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) , the cause of the novel and severe coronavirus disease, 2019 (COVID-19). Paxlovid is given orally for 5 days in patients early in the course of infection and has not been linked to serum aminotransferase elevations or to clinically apparent liver injury.
Background
Paxlovid consists of second generation protease inhibitor (nirmatrelvir) co-packaged with a pharmaceutical enhancer (ritonavir), which is used for oral treatment of recent-onset, mild-to-moderate COVID-19. Nirmatrelvir (ner mat” rel vir) is a peptidomimetic inhibitor of the main protease of SARS-CoV-2: Mpro and has antiviral activity in vitro against several coronaviruses including SARS-CoV-1 and -2. Ritonavir (ri toe’ na vir) is a protease inhibitor and potent inhibitor of the enzyme (CYP 3A4) responsible for the metabolism of nirmatrelvir, which allows for higher peak levels and more prolonged half-life of the active antiviral metabolite. In preregistration trials, Paxlovid started within 5 days of symptom onset demonstrated a 89% reduction in subsequent hospitalizations for COVID-19 (1.0% vs 6.7%) and a significant reduction in 28-day mortality (none vs 1.6%). Based upon these results and the ongoing COVID-19 pandemic, Paxlovid was granted Emergency Use Authorization (EUA) in December 2021 as therapy of nonhospitalized patients (adults and children 12 years or older) with documented COVID-19 infection who are at high risk of complications. Paxlovid is available under the EUA as tablets of 150 mg of nirmatrelvir co-packaged with 100 mg tablets of ritonavir; the recommended dose being 2 tablets of nirmatrelvir and one tablet of ritonavir twice daily for 5 days. Longer term therapy is not recommended, nor is therapy recommended for hospitalized patients or patients who have had symptoms or signs for more than 5 days. Currently, Paxlovid is being actively evaluated for efficacy and safety in treating patients not at high risk for complications, for children, and for patients with known exposure to COVID-19 (post-exposure prophylaxis). Paxlovid appears to be generally well tolerated; mild adverse events may include headache, myalgia, gastrointestinal upset, nausea and diarrhea. The total clinical experience with Paxlovid has been limited and its safety not fully defined.
Hepatotoxicity
In preregistration clinical trials, serum aminotransferase elevations were uncommon and mild, and were no more frequent with Paxlovid than with placebo. Furthermore, among more than 1000 patients treated with Paxlovid (nirmatrelvir 300 mg with ritonavir 100 mg twice daily) for 5 days in prelicensure studies, there were no reported episodes of clinically apparent liver injury. Confounding the issue is that serum aminotransferase elevations are common during symptomatic SARS-CoV-2 infection, present in up to 70% of patients and are more frequent in patients with severe disease and in those with the known risk factors for COVID-19 severity such as male sex, older age, higher body mass index and diabetes. Thus, Paxlovid has not been shown to cause liver injury, but the total clinical experience with its use is limited.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The lack of adverse events and hepatic injury from Paxlovid may be due to its relatively short duration of therapy. Paxlovid is metabolized by the cytochrome P450 system (largely CYP 3A4) and is given with a CYP 3A4 inhibitor to prolong its half-life and achieve better plasma concentrations and prolong its half-life. However, as a consequence Paxlovid is likely to have significant drug-drug interactions with agents that are metabolized by the CYP 3A4 enzyme. Whether longer term Paxlovid is also without serious adverse events remains to be seen.
Drug Class: Antiviral Agents
Other Drugs in the Subclass: Molnupiravir
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Nirmatrelvir and Ritonavir – Paxlovid®
DRUG CLASS
Antiviral Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
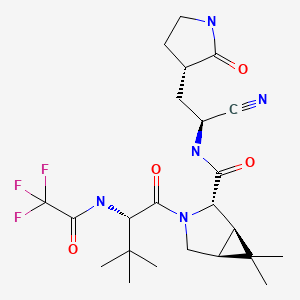
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Nirmatrelvir | 2628280-40-8 | C23-H32-F3-N5-O4 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 31 January 2022
Abbreviations used: COVID-19, coronavirus disease, 2019; ICU, intensive care unit; IFN, interferon; IL, interleukin; MERS, Middle East respiratory syndrome; NHC, N-hydroxycytidine; SARS-CoV-2, severe acute respiratory syndrome-coronavirus 2.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [PMC free article: PMC7159299] [PubMed: 31986264](Among 41 adults with COVID-19 pneumonia hospitalized in Wuhan China in December 2019-January 2020, 37% had serum AST elevations [62% of those in the ICU and 25% of those not] with concurrent elevations in proinflammatory cytokines [IL1B, IL6, IL2, IFN gamma], and 15% died).
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, et al. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. [PMC free article: PMC7092819] [PubMed: 32109013](Among1099 patients hospitalized with COVID-19 at 552 hospitals in China through January 2020, the median age was 47 years, 42% were women, 2.4% were admitted to an ICU, 1.4% died and ALT elevations arose in 4.1%).
- Qiu H, Wander P, Bernstein D, Satapathy SK. Acute on chronic liver failure from novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Liver Int. 2020;40:1590–1593. [PubMed: 32369658](56 year old woman with decompensated alcoholic cirrhosis developed worsening jaundice and liver function when admitted with SARS-CoV-2 infection [bilirubin rising from 9.4 to ~17.8 mg/dL, ALT from 94 to ~275 U/L, AST from 184 to ~880 U/L, Alk P from 128 to ~195 U/L, INR from 1.92 to 2.6], improving back towards baseline as the infection resolved).
- Wander P, Epstein M, Bernstein D. COVID-19 presenting as acute hepatitis. Am J Gastroenterol. 2020;115:941–942. [PMC free article: PMC7172489] [PubMed: 32301760](59 year old woman with HIV infection developed fatigue and jaundice [bilirubin 0.6 mg/dL, ALT 697 U/L, Alk P 145 U/L, INR 1.08] and then developed fever and cough with positive tests for SARS-CoV-1, liver tests falling over the next week as she recovered from COVID-19 ).
- Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal liver tests in COVID-19: A retrospective observational cohort study of 1827 patients in a major U.S. hospital network. Hepatology. 2020 Oct;72(4):1169–1176. [PMC free article: PMC9258788] [PubMed: 32725890](Among 1877 patients hospitalized with SARS-CoV-2 infection, serum ALT levels were elevated before hospitalization in 19%, at admission in 42% and a peak during hospitalization in 62% with 21% being greater than 5 times ULN; elevations correlated with disease severity and its risk factors: male sex, older age, higher BMI and diabetes).
- Owen DR, Allerton CMN, Anderson AS, Aschenbrenner L, Avery M, Berritt S, Boras B, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–1593. [PubMed: 34726479](Development and characterization of a small molecule inhibitor [PF-07321332] of the main protease of SARS-CoV-2, its antiviral activity in several cell culture system; its pharmacology, safety and activity in animal models; and its single dose tolerance and pharmacokinetics with and without CYP 3A4 inhibition [ritonavir] in humans).
- Chan HTH, Moesser MA, Walters RK, Malla TR, Twidale RM, John T, Deeks HM, et al. Discovery of SARS-CoV-2 Mpro peptide inhibitors from modelling substrate and ligand binding. Chem Sci. 2021;12:13686–13703. [PMC free article: PMC8549791] [PubMed: 34760153](Biophysical and crystallographic analyses of the main protease [Mpro] of SARS-CoV-2 and its 11 natural cleavage sites led to design of candidate synthetic peptides with high affinity for the catalytic site that might serve as potent, highly specific Mproinhibitors of potential use in therapy of COVID-19).
- Wang Z, Yang L. In the age of Omicron variant: Paxlovid raises new hopes of COVID-19 recovery. J Med Virol. 2021 Dec 22; Epub ahead of print. [PubMed: 34936106](Letter summarizing the promise held for Paxlovid as an effective therapy for SARS-CoV-2 infection; an orally available, second generation protease inhibitor similar to boceprevir which has activity against multiple coronaviruses including MERS and SARS-CoV-1 and multiple variants of SARS-CoV-2; 8 clinical trials are underway; antiviral resistant mutations in the protease being a critical issue for the future).
- Mahase E. COVID-19: Pfizer's Paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375(n2713) [PubMed: 34750163](News reports of the sponsor’s announcement of results of a double-blind, placebo-controlled trial of PF-07321332 [combined with ritonavir] in 1219 adults with confirmed SARS-CoV-2 infection who started treatment within 5 days of symptom onset, showing a reduction in subsequent hospitalization [1% vs 6.7%] and death [none vs 1.8%] from COVID-19).
- Couzin-Frankel J. Antiviral pills could change pandemic's course. Science. 2021;374(6569):799–800. [PubMed: 34762459](News report of the promise of antivirals including Paxlovid and molnupiravir as a means of treatment for early SARS-CoV-2 infection; no discussion of adverse events).
- Paxlovid for treatment of COVID-19. Med Lett Drug Ther. 2022;64:9–10. [PubMed: 35134040](Concise review of the mechanism of action, clinical efficacy, safety and indications of Paxlovid shortly after its Emergency Use Authorization in the US mentions only mild adverse events arising during the 5 day treatment including dysgeusia, diarrhea, hypertension, and myalgia; no mention of ALT levels or liver injury).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Optimizing the use of Paxlovid in clinical practice.[Drugs Today (Barc). 2022]Optimizing the use of Paxlovid in clinical practice.McCarthy MW. Drugs Today (Barc). 2022 Nov; 58(11):539-546.
- Review Paxlovid (Nirmatrelvir/Ritonavir): A new approach to Covid-19 therapy?[Biomed Pharmacother. 2023]Review Paxlovid (Nirmatrelvir/Ritonavir): A new approach to Covid-19 therapy?Hashemian SMR, Sheida A, Taghizadieh M, Memar MY, Hamblin MR, Bannazadeh Baghi H, Sadri Nahand J, Asemi Z, Mirzaei H. Biomed Pharmacother. 2023 Jun; 162:114367. Epub 2023 Feb 6.
- Efficacy and safety of Huashi Baidu granule plus Nirmatrelvir-Ritonavir combination therapy in patients with high-risk factors infected with Omicron (B.1.1.529): A multi-arm single-center, open-label, randomized controlled trial.[Phytomedicine. 2023]Efficacy and safety of Huashi Baidu granule plus Nirmatrelvir-Ritonavir combination therapy in patients with high-risk factors infected with Omicron (B.1.1.529): A multi-arm single-center, open-label, randomized controlled trial.Yu Z, Zheng Y, Chen B, Lv J, Zhu X, Shang B, Xv Y, Tao R, Yang Y, Cong J, et al. Phytomedicine. 2023 Nov; 120:155025. Epub 2023 Aug 16.
- Paxlovid mouth likely is mediated by activation of the TAS2R1 bitter receptor by nirmatrelvir.[Biochem Biophys Res Commun. 2023]Paxlovid mouth likely is mediated by activation of the TAS2R1 bitter receptor by nirmatrelvir.Caronia L, Xi R, Margolskee RF, Jiang P. Biochem Biophys Res Commun. 2023 Nov 19; 682:138-140. Epub 2023 Oct 2.
- Review The Design, Synthesis and Mechanism of Action of Paxlovid, a Protease Inhibitor Drug Combination for the Treatment of COVID-19.[Pharmaceutics. 2024]Review The Design, Synthesis and Mechanism of Action of Paxlovid, a Protease Inhibitor Drug Combination for the Treatment of COVID-19.Bege M, Borbás A. Pharmaceutics. 2024 Feb 2; 16(2). Epub 2024 Feb 2.
- Paxlovid - LiverToxPaxlovid - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...