NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Pirfenidone is an orally available pyridinone derivative that inhibits collagen formation and is used to treat idiopathic pulmonary fibrosis. Elevations in serum enzyme levels during pirfenidone therapy are not uncommon, but it has yet to be implicated in cases of clinically apparent liver injury with jaundice.
Background
Pirfenidone (pir fen' i done) is an orally available, antiinflammatory and antifibrotic agent that is used to treat idiopathic pulmonary fibrosis. It is a small molecular weight phenyl substituted pyridinone that has antifibrotic activity both in vitro and in vivo. In animal models, pirfenidone decreases fibroblast proliferation and reduces transforming growth factor-beta (TGF-β) synthesis and activation of fibrogenic pathways. In several prospective, placebo controlled trials, pirfenidone was found to reduce the progression of fibrosis and worsening of lung function in patients with idiopathic pulmonary fibrosis. Pirfenidone was approved for use in the United States in 2014, but has been available in other countries for much longer. It is available as capsules of 267 mg under the brand name Esbriet. The typical initial dose in adults is one capsule (267 mg) orally three times daily, which can be increased to 3 capsules three times daily based upon tolerance. Side effects are not uncommon, but are generally mild and can include photosensitivity, rash and gastrointestinal upset with nausea, diarrhea, dyspepsia, reflux and abdominal pain.
Hepatotoxicity
In large randomized controlled trials, serum aminotransferase elevations more than 3 times the upper limit of normal (ULN) occurred in 4% of pirfenidone- compared to less than 1% of placebo-treated patients. The elevations were generally asymptomatic and short lived, resolving with or without dose modification and requiring drug discontinuation in approximately 1% of patients. Despite the frequency of serum enzyme elevations during therapy, clinically apparent liver injury was not reported in preregistration studies. Nevertheless, since the general availability of pirfenidone in the United States and during years of clinical use elsewhere, there have been isolated case reports of clinically apparent liver injury due to pirfenidone, some of which were severe and even fatal. The latency to onset ranged from one month to one year and the injury was usually hepatocellular or mixed. Immunoallergic features were not common.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which pirfenidone might cause liver injury is not known. It is metabolized in the liver largely via the cytochrome P450 system, predominantly CYP 1A2, and liver injury may be due to production of a toxic or immunogenic metabolite. Pirfenidone is also susceptible to drug-drug interactions with strong inducers or inhibitors of CYP 1A2 (such as fluvoxamine).
Outcome and Management
While chronic therapy with pirfenidone can be associated with mild-to-moderate serum aminotransferase elevations, it has only rarely been linked to cases of clinically apparent liver injury. Nevertheless, monitoring of serum aminotransferase levels monthly during the first 6 months and every 3 months thereafter is recommended. Patients who develop aminotransferase elevations on therapy should be monitored more carefully, and pirfenidone should be permanently discontinued if jaundice or symptoms of liver injury arise or if serum ALT or AST levels rise above 5 times the ULN.
Drug Class: Pulmonary Fibrosis Agents
Other Drugs in the Class: Nintedanib
CASE REPORT
Case 1. Fatal, acute liver injury attributed to pirfenidone.(1)
An 77 year old man with Parkinson disease and idiopathic pulmonary fibrosis developed anorexia and nausea within 2 to 3 weeks of starting pirfenidone (200 mg three times daily), which was followed in the next 1 to 2 weeks by dark urine, jaundice and altered sensorium. He had no history of liver disease, alcohol abuse or risk factors for viral hepatitis. He had been diagnosed with Parkinson disease 5 years previously and had been on a stable dose of levodopa/carbidopa for years. He denied taking other medications, over-the-counter drugs or herbal products and had no history of drug allergy. His liver test results had been normal in the past. On presentation, he was jaundiced, tachypneic, hypotensive and mildly encephalopathic. Laboratory testing showed total bilirubin of 12.8 mg/dL (direct 7.8), ALT 526 U/L, AST 1260 U/L, Alk P 434 U/L and LDH 687 U/L [R= 4.0]. The INR was 2.1 and serum ammonia 330 µmol/L. He had elevations in serum creatinine [2.1 mg/dL] and blood lactate [6.1 mmol/L] with severe acidosis [pH 7.04]. He was admitted to the intensive care unit and treated with N-acetyl cysteine. Tests for acute hepatitis A, B, C and E were negative as were markers for cytomegalovirus, Epstein Barr virus and herpes virus infection. Routine autoantibodies were undetectable and immunoglobulin levels were normal. Imaging of the liver showed no evidence of biliary obstruction. He rapidly developed multiorgan failure and died within 4 days of admission. Autopsy showed confluent and bridging necrosis, collapse and few surviving, swollen and vacuolated hepatocytes, no fibrosis and mild portal inflammation suggestive of acute liver failure due to drug induced liver injury.
Key Points
| Medication: | Pirfenidone (600 mg daily) |
|---|---|
| Pattern: | Hepatocellular (R=9.6) |
| Severity: | 5+ (jaundice, hospitalization, death) |
| Latency: | 3-4 weeks |
| Recovery: | None |
| Other medications: | Levodopa/carbodopa for several years |
Comment
This was a dramatic example of acute liver failure arising in an elderly man who had recently started pirfenidone. The precipitous onset and course suggests that shock and hypoxia may have contributed to the severity of the injury and fatal outcome. While instances of serum aminotransferase elevations without jaundice have been reported with pirfenidone therapy, there have been no instances of moderate injury with jaundice and a self-limited course reported in the literature. Nevertheless, this case is somewhat convincing. No other cause of jaundice was found despite extensive testing and the autopsy was consistent with drug induced liver injury. The product label for pirfenidone states that cases of hepatitis, jaundice and fatal hepatotoxicity during pirfenidone therapy have been reported to the sponsor. Furthermore, it recommends regular monitoring of liver tests before and monthly for the first 6 months of treatment. In this case, the liver injury arose during the first month of treatment and would not have been prevented by such screening. Furthermore, the capsule size used was lower than the standard (200 mg rather than 267 mg amounts) and there was no dose escalation to 3 capsules 3 times daily as is recommended in treatment of patients with idiopathic pulmonary fibrosis.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Pirfenidone – Esbriet®
DRUG CLASS
Pulmonary Fibrosis Agents
Product labeling at DailyMed, National Library of Medicine, NIH
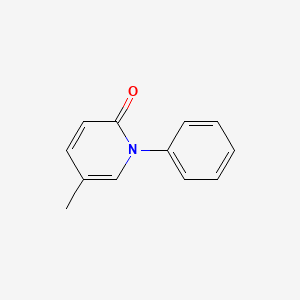
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Pirfenidone | 53179-13-8 | C12-H11-N-O |
|
CITED REFERENCE
- 1.
- Verma N, Kumar P, Mitra S, Taneja S, Dhooria S, Das A, Duseja A, et al. Drug idiosyncrasy due to pirfenidone presenting as acute liver failure: Case report and mini-review of the literature. Hepatol Commun. 2017;2:142–7. [PMC free article: PMC5796329] [PubMed: 29404521]
ANNOTATED BIBLIOGRAPHY
References updated: 24 June 2020
- Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013.(Multi-authored textbook of hepatotoxicity published in 2013; does not discuss pirfenidone).
- Angulo P, MacCarty RL, Sylvestre PB, Jorgensen RA, Wiesner RH, LaRusso NA, Lindor KD. Pirfenidone in the treatment of primary sclerosing cholangitis. Dig Dis Sci. 2002;47:157–61. [PubMed: 11837718](Among 24 patients with primary sclerosing cholangitis treated with pirfenidone [2400 mg daily] for 1 year, AST and Alk P levels, histology and cholangiography findings did not change significantly, while adverse events were frequent and 1 patient had worsening of liver disease after 6 months of therapy leading to referral for liver transplantation).
- Gahl WA, Brantly M, Troendle J, Avila NA, Padua A, Montalvo C, Cardona H, et al. Effect of pirfenidone on the pulmonary fibrosis of Hermansky-Pudlak syndrome. Mol Genet Metab. 2002;76:234–42. [PubMed: 12126938](Among 21 adults with Hermansky-Pudlak syndrome and progressive pulmonary fibrosis treated with pirfenidone or placebo for up to 3 years, serum ALT levels did not change and there were no serious hepatic adverse events).
- Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, Taguchi Y, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–7. [PubMed: 15665326](Among 107 patients with idiopathic pulmonary fibrosis treated with pirfenidone or placebo for 6 months, one patient discontinued therapy because of abnormal liver tests and one developed a liver cancer, both of whom were on pirfenidone).
- Armendáriz-Borunda J, Islas-Carbajal MC, Meza-García E, Rincón AR, Lucano S, Sandoval AS, Salazar A, et al. A pilot study in patients with established advanced liver fibrosis using pirfenidone. Gut. 2006;55:1663–5. [PMC free article: PMC1860119] [PubMed: 17047115](Among 15 patients with chronic hepatitis C treated with pirfenidone for 12 months [1200 mg daily], ALT levels declined and paired liver biopsies showed improvements in fibrosis scores in 5 and stable scores in 10 patients).
- Rockey DC. Current and future anti-fibrotic therapies for chronic liver disease. Clin Liver Dis. 2008;12:939–62. [PMC free article: PMC2610449] [PubMed: 18984475](Review of the pathogenesis and cellular pathways of fibrosis in patients with chronic liver disease, and the status of antifibrotic agents, none of which have been shown to be effective in treating or preventing hepatic fibrosis).
- Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, Taguchi Y, et al. Pirfenidone Clinical Study Group in Japan. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–9. [PubMed: 19996196](Among 275 patients with idiopathic pulmonary fibrosis treated with pirfenidone [1200 or 1800 mg daily] or placebo for 12 months, the decline in lung vital capacity was less with pirfenidone [-0.08 and -0.09] than placebo [-0.16]; photosensitivity occurred in 51-52% on pirfenidone and GGT elevations in 22-23%; no mention of clinically apparent liver injury).
- O'Brien K, Troendle J, Gochuico BR, Markello TC, Salas J, Cardona H, Yao J, et al. Pirfenidone for the treatment of Hermansky-Pudlak syndrome pulmonary fibrosis. Mol Genet Metab. 2011;103:128–34. [PMC free article: PMC3656407] [PubMed: 21420888](Among 35 patients with Hermansky-Pudlak syndrome and pulmonary fibrosis who were treated with pirfenidone [n=23] or placebo [n-12] for 1-3 years, mean serum ALT levels did not change and "there was no evidence of ... hepatic toxicity").
- Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, King TE Jr, et al. CAPACITY Study Group. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–9. [PubMed: 21571362](Among 779 patients with pulmonary fibrosis treated in two studies with pirfenidone [1197 or 2403 mg daily] or placebo, side effects included nausea [36%], dyspepsia [19%], anorexia [11%], photosensitivity [12%], rash [32%] and ALT elevations above 3 times ULN [4% vs <1% in controls]).
- Two new drugs for idiopathic pulmonary fibrosis. Med Lett Drugs Ther. 2014;56(1457):123–4. [PubMed: 25461229](Concise review of the mechanism of action, efficacy and safety of pirfenidone and nintedanib for idiopathic pulmonary fibrosis mentions that both agents can increase hepatic enzyme levels and dose adjustment may be required).
- King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, et al. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–92. [PubMed: 24836312](Among 555 patients with idiopathic pulmonary fibrosis treated with pirfenidone [2403 mg/day] or placebo for 52 weeks, pirfenidone led to a lower rate of disease progression, but a higher rate of adverse events including ALT elevations above 3 times ULN in 2.9% [vs 0.7% in placebo controls], but all elevations "were reversible and without clinically significant consequences").
- Valeyre D, Albera C, Bradford WZ, Costabel U, King TE Jr, Leff JA, Noble PW, Sahn SA, et al. Comprehensive assessment of the long-term safety of pirfenidone in patients with idiopathic pulmonary fibrosis. Respirology. 2014;19:740–7. [PMC free article: PMC4230393] [PubMed: 24836849](Among 789 patients with idiopathic pulmonary fibrosis being monitored for safety for an average of 2.6 years, elevated ALT or AST values [above 3 times ULN] occurred in 2.7% on pirfenidone, but none developed clinically apparent liver injury, although 1% required drug discontinuation for the enzyme elevations).
- Cottin V, Maher T. Long-term clinical and real-world experience with pirfenidone in the treatment of idiopathic pulmonary fibrosis. Eur Respir Rev. 2015;24(135):58–64. Erratum in Eur Respir Rev 2015; 24(137): 545. [PMC free article: PMC9487763] [PubMed: 25726556](Analysis of long term, postmarketing monitoring of pirfenidone therapy of idiopathic pulmonary fibrosis focused on the frequency of common adverse events, including nausea [32%], diarrhea [19%], photosensitivity [9%] and rash [26%]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to pirfenidone or other agents for pulmonary fibrosis).
- Khanna D, Albera C, Fischer A, Khalidi N, Raghu G, Chung L, Chen D, et al. An open-label, phase II study of the safety and tolerability of pirfenidone in patients with scleroderma-associated interstitial lung disease: the LOTUSS Trial. J Rheumatol. 2016;43(9):1672–9. [PubMed: 27370878](Among 63 patients with scleroderma and interstitial lung disease treated with pirfenidone in two dose escalation regimens for a total of 16 weeks, adverse events were common [96.8%] and mostly nausea, headache and fatigue; “minor elevations in liver function tests were sporadic and not considered clinically relevant” and ALT levels remained below 3 times ULN).
- Lancaster L, Morrison L, Auais A, Ding B, Iqbal A, Polman B, Flaherty KR. Safety of pirfenidone in patients with idiopathic pulmonary fibrosis: experience from 92 sites in an open-label US expanded access program. Pulm Ther. 2017;3:317–25. [PMC free article: PMC6964201] [PubMed: 32026347](Among 1620 patients with idiopathic pulmonary fibrosis treated with pirfenidone in an expanded access program for an average of 23 weeks, adverse events occurred in 65%, which were severe in 3% and life-threatening in 0.2%, the most common adverse events being nausea [23%] and fatigue [20%] with ALT elevations above 5 times ULN in 13 patients [0.8%] usually by week 8, and hepatic enzyme elevations being considered severe in 10 [0.6%]).
- Verma N, Kumar P, Mitra S, Taneja S, Dhooria S, Das A, Duseja A, et al. Drug idiosyncrasy due to pirfenidone presenting as acute liver failure: Case report and mini-review of the literature. Hepatol Commun. 2017;2:142–7. [PMC free article: PMC5796329] [PubMed: 29404521](77 year old man with Parkinson disease and idiopathic pulmonary fibrosis developed jaundice 3-4 weeks after starting pirfenidone [bilirubin 12.8 mg/dL, ALT 526 U/L, Alk P 434 U/L, INR 2.1], with lactic acidosis and hepatic encephalopathy and progressive multiorgan failure dying 4 days after hospital admission: Case 1).
- Benesic A, Jalal K, Gerbes AL. Acute liver failure during pirfenidone treatment triggered by co-medication with esomeprazole. Hepatology. 2019;70:1869–71. [PubMed: 31034631](75 year old man with idiopathic pulmonary fibrosis developed liver test abnormalities a year after starting pirfenidone and 3 days after starting omeprazole for gastroesophageal reflux [bilirubin 6.4 mg/dL, ALT 973U/L, Alk P 379 U/L, INR 1.24], with progressive worsening despite stopping both drugs [bilirubin rising to 23.6 mg/dL], leading to hepatic and multiorgan failure and death 24 days after presentation).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Nintedanib.[LiverTox: Clinical and Researc...]Review Nintedanib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials.[Lancet. 2011]Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials.Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, King TE Jr, Lancaster L, Sahn SA, Szwarcberg J, et al. Lancet. 2011 May 21; 377(9779):1760-9. Epub 2011 May 13.
- Pirfenidone Inhibits Cell Proliferation and Collagen I Production of Primary Human Intestinal Fibroblasts.[Cells. 2020]Pirfenidone Inhibits Cell Proliferation and Collagen I Production of Primary Human Intestinal Fibroblasts.Cui Y, Zhang M, Leng C, Blokzijl T, Jansen BH, Dijkstra G, Faber KN. Cells. 2020 Mar 22; 9(3). Epub 2020 Mar 22.
- Real-life experiences in a single center: efficacy of pirfenidone in idiopathic pulmonary fibrosis and fibrotic idiopathic non-specific interstitial pneumonia patients.[Ther Adv Respir Dis. 2020]Real-life experiences in a single center: efficacy of pirfenidone in idiopathic pulmonary fibrosis and fibrotic idiopathic non-specific interstitial pneumonia patients.Feng H, Zhao Y, Li Z, Kang J. Ther Adv Respir Dis. 2020 Jan-Dec; 14:1753466620963015.
- Review Pirfenidone: a novel agent for the treatment of idiopathic pulmonary fibrosis.[Ann Pharmacother. 2013]Review Pirfenidone: a novel agent for the treatment of idiopathic pulmonary fibrosis.Potts J, Yogaratnam D. Ann Pharmacother. 2013 Mar; 47(3):361-7. Epub 2013 Feb 12.
- Pirfenidone - LiverToxPirfenidone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...