NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Primaquine is an aminoquinoline that has been used for the prevention and therapy of malaria for more than 50 years. Primaquine is not associated with serum enzyme elevations during therapy and has yet to be linked to instances of clinically apparent acute liver injury.
Background
Primaquine (prim' a kwin) is a synthetic aminoquinoline that acts by binding to the protozoal or parasitic DNA and preventing DNA and RNA production and subsequent protein synthesis; it is active against several of the stages in the development of the plasmodia including liver schizonts, hypnozoites and gametocytes and has most activity against Plasmodium ovale and vivax. Primaquine was approved for use in the United States in 1952. The current indications are for treatment of vivax malaria in combination with other antimalarial agents. It is also used as a second line agent in the prophylaxis against vivax malaria. Primaquine has been proposed as a major agent to prevent relapse after therapy of acute attacks of vivax malaria with more schizontocidal agents such as chloroquine or artemesinin. Primaquine is available in generic forms as tablets of 26.3 mg containing 15 mg of primaquine base. The recommended dosage for prophylaxis in adults is 30 mg of the base once daily starting before travel and continuing for at least 7 days after return from an endemic area. The dosage for therapy and prevention of relapse is usually 15 mg daily for 14 days or 45 mg once weekly for 8 weeks. Specific recommendations on the therapy of malaria including details on diagnosis, drug dosage and safety are available at the CDC website: http://www.cdc.gov/malaria/. Common side effects of primaquine include headache, anorexia, nausea, diarrhea, abdominal pain, skin rash and itching.
Hepatotoxicity
Despite use for more than 50 years, primaquine has not been linked to significant serum aminotransferase elevations or to clinically apparent acute liver injury. Primaquine can cause hemolysis in patients with G6PD deficiency, which can result in mild jaundice.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Hepatic reactions to quinine are usually due to hypersensitivity reactions, but have been rarely reported with primaquine. Primaquine undergoes metabolism by the liver to its active metabolite that is excreted unchanged in the urine.
Outcome and Management
There does not seem to be cross reactivity to hepatic injury among the various antimalarial agents and switching to other drug can be done.
Drug Class: Antimalarial Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Primaquine – Generic
DRUG CLASS
Antimalarial Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Primaquine | 63-45-6 | C15-H21-N3-O.2H3-O4-P |
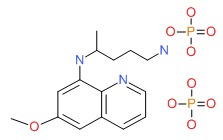 |
ANNOTATED BIBLIOGRAPHY
References updated: 02 February 2017
- Zimmerman HJ. Antiprotozoal agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 623-5.(Expert review of hepatotoxicity published in 1999; states that primaquine has not been linked to instances of clinically apparent liver disease).
- Vinetz JM, Clain J, Bounkeua V, Eastman RT, Fidock D. Chemotherapy of malaria.l In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman.s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1383-418.(Textbook of pharmacology and therapeutics).
- Thornsvard CT, Guider BA, Kimball DB. An unusual reaction to chloroquine-primaquine. JAMA 1976; 235: 1719-20. [PubMed: 946467](39 year old woman developed fever, abdominal pain, myalgias and red urine 2 days after starting chloroquine-primaquine prophylaxis [bilirubin 0.8 mg/dL, AST >300 U/L, Alk P 60 U/L]; porphyrin testing indicated porphyria cutanea tarda).
- Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf 2004; 27: 25-61. [PubMed: 14720085](Review of the toxicities and side effects of antimalarials; quinine can cause a characteristic hypersensitivity reaction with liver injury; chloroquine can cause worsening of acute porphyria; primaquine can cause hemolysis, but has not been linked to cases of hepatitis).
- Leslie T, Mayan I, Mohammed N, Erasmus P, Kolaczinski J, Whitty CJ, Rowland M. A randomised trial of an eight-week, once weekly primaquine regimen to prevent relapse of plasmodium vivax in Northwest Frontier Province, Pakistan. PLoS One 2008; 3: e2861. [PMC free article: PMC2481394] [PubMed: 18682739](Among 200 patients with vivax malaria infection randomized to receive two regimens of primaquine in addition to chloroquine therapy for vivax malaria infection, primaquine reduced failure rates and there were "no serious or notable non-serious adverse events" except for anemia in one G6PD deficient patient).
- Ebringer A, Heathcote G, Baker J, Waller M, Shanks GD, Edstein MD. Evaluation of the safety and tolerability of a short higher-dose primaquine regimen for presumptive anti-relapse therapy in healthy subjects. Trans R Soc Trop Med Hyg. 2011 Oct; 105 (10): 568-73. [PubMed: 21890160](Among 203 Australian soldiers treated with primaquine as a higher dose 7 day regimen, there were no "clinically significant differences ... in biochemical indices before and after treatment").
- John GK, Douglas NM, von Seidlein L, Nosten F, Baird JK, White NJ, Price RN. Primaquine radical cure of Plasmodium vivax: a critical review of the literature. Malar J 2012; 11: 280. [PMC free article: PMC3489597] [PubMed: 22900786](Review of the activity, efficacy and safey of primaquine as therapy and means of prevention of relapse of vivax malaria).
- Baird KJ, Maguire JD, Price RN. Diagnosis and treatment of Plasmodium vivax malaria. Adv Parasitol 2012; 80: 203-70. [PubMed: 23199489](Extensive review of the history and modern approach to diagnosis and treatment of vivax malaria; primaquine has been shown to be effective in preventing relapse and might play a role in the reaching of a goal of elimination of malaria transmission; side effects are few and usually minor except in persons with G6PD deficiency, a problem for the developing world).
- Sutanto I, Tjahjono B, Basri H, Taylor WR, Putri FA, Meilia RA, Setiabudy R, et al. Randomized, open-label trial of primaquine against vivax malaria relapse in Indonesia. Antimicrob Agents Chemother 2013; 57: 1128-35. [PMC free article: PMC3591862] [PubMed: 23254437](Prospective trial of three regimens for vivax malaria among 116 Indonesian soliders; primaquine was 92-98% effective in preventing relapse and "most laboratory findings" including serum ALT and alkaline phosphatase levels remained normal before, during and after treatment).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to an antimalarial agent).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. (In a. [PubMed: 23419359]population based study from Iceland, 96 cases of drug induced liver injury were identified over a 2 year period [2010 and 2011], but none were attributed to an antimalarial agent).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to an antimalarial agentl).
- Advice for travelers. Treat Guidel Med Lett 2015: 57 (1466): 52-8.(Concise guidelines on prevention of malaria in travelers indicates that primaquine is an appropriate prophylactic agent for patients who cannot tolerate chloroquine).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Tafenoquine.[LiverTox: Clinical and Researc...]Review Tafenoquine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Mass administration of an antimalarial drug combining 4-aminoquinoline and 8-aminoquinoline in Tanganyika.[Bull World Health Organ. 1962]Mass administration of an antimalarial drug combining 4-aminoquinoline and 8-aminoquinoline in Tanganyika.CLYDE DF. Bull World Health Organ. 1962; 27(2):203-12.
- Primaquine at alternative dosing schedules for preventing relapse in people with Plasmodium vivax malaria.[Cochrane Database Syst Rev. 2019]Primaquine at alternative dosing schedules for preventing relapse in people with Plasmodium vivax malaria.Milligan R, Daher A, Graves PM. Cochrane Database Syst Rev. 2019 Jul 5; 7(7):CD012656. Epub 2019 Jul 5.
- Single dose tafenoquine for preventing relapse in people with plasmodium vivax malaria-an updated meta-analysis.[Travel Med Infect Dis. 2020]Single dose tafenoquine for preventing relapse in people with plasmodium vivax malaria-an updated meta-analysis.Anjum MU, Naveed AK, Mahmood SN, Naveed OK. Travel Med Infect Dis. 2020 Jul-Aug; 36:101576. Epub 2020 Feb 6.
- Review Primaquine pharmacology in the context of CYP 2D6 pharmacogenomics: Current state of the art.[Pharmacol Ther. 2016]Review Primaquine pharmacology in the context of CYP 2D6 pharmacogenomics: Current state of the art.Marcsisin SR, Reichard G, Pybus BS. Pharmacol Ther. 2016 May; 161:1-10. Epub 2016 Mar 22.
- Primaquine - LiverToxPrimaquine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...