NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The serotonin type 3 (5-HT3) receptor antagonists are potent antiemetics used for prevention of postsurgical or chemotherapy induced nausea and vomiting and for some agents as therapy of diarrhea-predominant irritable bowel syndrome. The 5-HT3 receptor antagonists are associated with a low rate of transient serum enzyme elevations during therapy, but have been only rarely implicated in cases of clinically apparent liver injury.
Background
The 5-HT3 receptor antagonists act to block the effects of serotonin on specific receptors that are found most frequently in the gastrointestinal tract and the central nervous system. The antiemetic effects appear to be the result of both central and peripheral inhibition of serotonin activity, with a decrease in vagal activity as well as interruption of pathways in the chemoreceptor trigger zone and solitary tract nucleus of the brainstem. 5-HT3 receptor antagonists in clinical use for treatment of nausea and vomiting in the United States include (with brand name and year of approval) granisetron (Kytril: 1994), dolasetron (Anzemet: 1997), ondansetron (Zofran: 1999), and palonosetron (Aloxi: 2003). All except palonosetron are also available in generic forms. These four 5-HT3 receptor antagonists are approved for prevention of nausea and vomiting after major surgery, chemotherapy or radiation therapy. They are sometimes used off-label to treat severe nausea and vomiting including that from hyperemesis gravidarum and acute gastroenteritis. They are not effective for motion sickness. These agents are generally given intravenously before or at the time of chemotherapy or surgery, but are also available as oral tablets, capsules and solutions, but are usually given for 1 to 3 days only. Common side effects include headache, fatigue, dizziness and constipation.
Alosetron (al oh’ se tron) is a 5-HT3 receptor blocker, but was developed and used largely for management of diarrhea-predominant irritable bowel syndrome rather than as an antiemetic. Its introduction in the 1990s however, was followed by cases of severe, even life-threatening, constipation which led to its withdrawal in 2000. Alosetron was reintroduced in 2002 with restrictions on its availability and limitation to women with severe diarrhea-predominant irritable bowel syndrome. Alosetron is available as 0.5 and 1.0 mg tablets generically and under the brand name Lotronex. The typical initial dose in adults is 0.5 mg twice daily, which can be increased to 1 mg twice daily if it is well tolerated. Side effects include abdominal pain and constipation, which can be severe resulting in intestinal obstruction, ileus, impaction and ischemic colitis. Patients treated with alosetron must be enrolled in a prescribing program and have regular monitoring for side effects.
Dolasetron (doe las’ e tron) is a 5-HT3 receptor blocker used as an antiemetic to prevent nausea and vomiting postoperatively or after cancer chemotherapy. It was first approved in 1997 and is available as tablets of 50 and 100 mg and as a solution for injection in single or multidose vials (20 mg/mL) under the brand name Anzemet. As with most 5-HT3 receptor blockers, the dose varies by mode of administration, indication and expected severity and duration of nausea. Side effects include headache, fatigue, diarrhea and dizziness. Dolasetron can prolong the QTc interval and is susceptible to drug-drug interactions.
Granisetron (gra nis’ e tron) is a 5-HT3 receptor blocker used as an antiemetic to prevent nausea and vomiting postoperatively or after cancer chemotherapy. Introduced in 1993, granisetron is available as tablets of 1 mg and as a solution for injection in single or multidose vials (1 mg/mL) generically and under the brand name Kytril. A transdermal patch of granisetron for prevention of chemotherapy associated nausea and vomiting is also available (Sancuso). As with other 5-HT3 receptor blockers, the dose varies by mode of administration, indication and expected severity and duration of nausea. Side effects of granisetron can include headache, fatigue, diarrhea and dizziness.
Ondansetron (on dan’ se tron) is a 5-HT3 receptor blocker that was developed as an antiemetic and was introduced in 1993, the first of this class of anti-emetic agents. The current indications are for prevention of nausea and vomiting postoperatively or after cancer chemotherapy. Ondansetron is available as tablets of 4, 8, 16 and 24 mg and as a solution for injection in single or multidose vials (2 mg/mL) in multiple generic forms and under the brand name Zofran. As with other 5-HT3 receptor blockers, the dose varies by mode of administration, indication and expected severity and duration of nausea. Side effects include headache, fatigue, diarrhea and dizziness. Ondansetron can prolong the QTc interval.
Palonosetron (pal” oh noe’ se tron) is a 5-HT3 receptor blocker that was developed as an antiemetic and introduced in 2003. The current indications are prevention of nausea and vomiting postoperatively or after cancer chemotherapy. Palonosetron is available as capsules of 0.5 mg and as a solution for injection in single or multidose vials (0.05 mg/mL) generically and under the brand name Aloxi. As with other 5-HT3 receptor blockers, the dose varies by mode of administration, indication and expected severity and duration of nausea. Side effects include headache, fatigue, diarrhea and dizziness. Palonosetron has not been shown to prolong the QTc interval.
Hepatotoxicity
The 5-HT3 receptor antagonists have been linked to occasional instances of serum enzyme elevations during therapy, but these are generally mild and asymptomatic, resolving rapidly. Because they are used at the time of surgery and with chemotherapy, instances of liver injury arising after their use have been reported, but other drugs or factors may have played a role in the published cases. The rate of serum enzyme elevations with 5-HT3 receptor antagonist therapy has ranged from 1% to 8% and has generally been no greater than that observed with placebo therapy. While moderate serum enzyme elevations during 5-HT3 receptor antagonist therapy have been described, there have been have been only rare and isolated reports of clinically apparent acute liver injury with jaundice attributed to these agents. The onset of injury has been within 1 to 2 weeks of exposure and the pattern of injury hepatocellular and without immunoallergic or autoimmune features. Instances of recurrence after re-exposure have been published. No instances of acute liver failure, chronic hepatitis or vanishing bile duct syndrome have been attributed to the 5-HT3 receptor antagonists.
Alosetron likelihood score: D (possible cause of clinically apparent liver injury).
Dolasetron likelihood score: E (unlikely cause of clinically apparent liver injury).
Granisetron likelihood score: E (unlikely cause of clinically apparent liver injury).
Ondansetron likelihood score: D (possible cause of clinically apparent liver injury).
Palonosetron likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The 5-HT3 receptor blockers are metabolized in the liver, largely via the cytochrome P450 system, but appear to have a low potential for causing liver injury. All except alosetron are used in low doses for short periods of time which may account for their relative lack of hepatotoxicity.
Outcome and Management
Serum aminotransferase elevations above 5 times the upper limit of normal (if confirmed) should lead to dose reduction or temporary cessation. Clinically apparent liver injury due to the 5-HT3 receptor blockers is rare, generally mild and self-limited and frequently arises only after the agent is discontinued. Rechallenge with the same agent can lead to reoccurrence but in some instances patients have tolerated other 5-HT3 receptor blockers without reappearance of liver injury.
Drug Class: Gastrointestinal Agents, Antiemetics
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Alosetron – Generic, Lotronex®
Dolasetron – Generic, Anzemet®
Granisetron – Generic, Kytril®
Ondansetron – Generic, Zofran®
Palonosetron – Aloxi®
DRUG CLASS
Gastrointestinal Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Alosetron | 122852-42-0 | C17-H18-N4-O |
 |
| Dolasetron | 115956-12-2 | C19-H20-N2-O3 |
 |
| Granisetron | 109889-09-0 | C18-H24-N4-O |
 |
| Ondansetron | 99614-02-5 | C18-H19-N3-O |
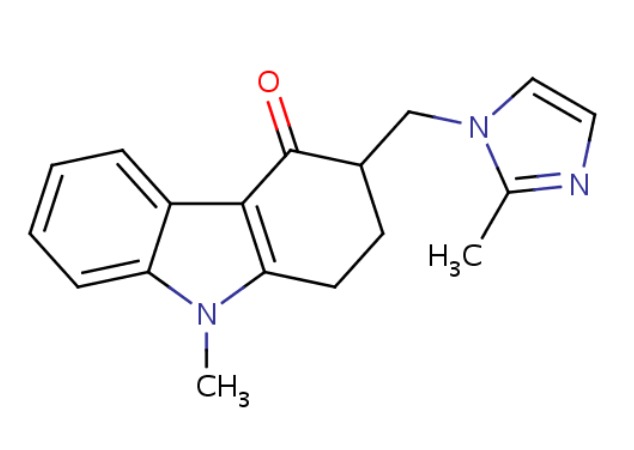 |
| Palonosetron | 135729-61-2 | C19-H24-N2-O |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 15 January 2018
- Zimmerman HJ. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 717-8.(Expert review of hepatotoxicity published in 1999, does not discuss the serotonin type 3 receptor antagonists).
- Sharkey KA, Wallace JL. Treatment of disorders of bowel motility and water flux: anti-emetics; agents used in biliary and pancreatic disease. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1323-50.(Textbook of pharmacology and therapeutics).
- Smith RN. Safety of ondansetron. Eur J Cancer Clin Oncol 1989; 25 Suppl 1: S47-50; discussion S51-4. [PubMed: 2533899](Review of safety of ondansetron mentions that liver test abnormalities have been observed on therapy, but were likely due to underlying cancer, metastases or chemotherapy).
- Hainsworth J, Harvey W, Pendergrass K, Kasimis B, Oblon D, Monaghan G, Gandara D, et al. A single-blind comparison of intravenous ondansetron, a selective serotonin antagonist, with intravenous metoclopramide in the prevention of nausea and vomiting associated with high-dose cisplatin chemotherapy. J Clin Oncol 1991; 9: 721-8. [PubMed: 1826739](Among 307 patients receiving cisplatin chemotherapy, ALT elevations above twice ULN occurred in 6% of those receiving ondansetron vs 0% on metoclopramide for prevention of nausea and vomiting, but all abnormalities were asymptomatic and resolved within 2 weeks).
- Finn AL. Toxicity and side effects of ondansetron. Semin Oncol 1992; 19 (4 Suppl 10): 53-60. [PubMed: 1387251](Analysis of the preclinical and early clinical evaluation of ondansetron mentions that ALT elevations [at least twice ULN] occurred in 2% to 8% of patients receiving ondansetron during cisplatin chemotherapy, but only 1% receiving the agent for postoperative nausea and vomiting, a rate similar to that with placebo).
- Sledge GW Jr, Einhorn L, Nagy C, House K. Phase III double-blind comparison of intravenous ondansetron and metoclopramide as antiemetic therapy for patients receiving multiple-day cisplatin-based chemotherapy. Cancer 1992; 70: 2524-8. [PubMed: 1423181](Among 45 patients with cancer receiving cisplatin based chemotherapy, 1 of 22 patients who were treated with metoclopramide versus 3 of 23 on ondansetron developed serum ALT elevations, but none developed signs of symptoms of liver injury).
- Chen M, Tanner A, Gallo-Torres H. Anaphylactoid-anaphylactic reactions associated with ondansetron. Ann Intern Med 1993; 119: 862. [PubMed: 8379613](Letter from the FDA summarizing 24 reports of anaphylactic reactions to ondansetron, occurring after 1-3 courses and marked by urticaria, angioedema, hypotension, bronchospasm and dyspnea, at least one case of which was fatal).
- Cersosimo RJ. Hepatotoxicity associated with cisplatin chemotherapy. Ann Pharmacother 1993; 27: 438-41. [PubMed: 8477119](69 year old man developed serum aminotransferase elevations after each of three courses of chemotherapy with cisplatin, peak ALT being lower with each course [203, 77 and 56 U/L], peaking on day 2 to 3 and falling to normal by day 10, with ondansetron given during the second and third course).
- Verrill M, Judson I. Jaundice with ondansetron. Lancet 1994; 344 (8916): 190-1. [PubMed: 7912780](35 year old man developed jaundice 10 days after two 8 mg intravenous doses of ondansetron [bilirubin 8.8 mg/dL, ALT 153 U/L, Alk P 77 U/L], resolving within 8 days; jaundice with fever recurring 9 days after reexposure [bilirubin 9.0 mg/dL], but not recurring when given granisetron).
- Wilde MI, Markham A. Ondansetron. A review of its pharmacology and preliminary clinical findings in novel applications. Drugs 1996; 52:773-94. [PubMed: 9118822](Review of the structure, mechanism of action, pharmacokinetics, efficacy and safety of ondansetron as an antiemetic agent mentions that abnormalities of liver tests have been associated with ondansetron therapy and severe symptomatic jaundice has been reported in one case).
- Chevallier B, Cappelaere P, Splinter T, Fabbro M, Wendling JL, Cals L, Catimel G, et al. A double-blind, multicentre comparison of intravenous dolasetron mesilate and metoclopramide in the prevention of nausea and vomiting in cancer patients receiving high-dose cisplatin chemotherapy. Support Care Cancer 1997; 5: 22-30. [PubMed: 9010986](Among 226 patients with cancer receiving cisplatin, 4% of 157 receiving dolasetron vs 1% receiving metoclopramide had liver test abnormalities during treatment).
- Balfour JA, Goa KL. Dolasetron. A review of its pharmacology and therapeutic potential in the management of nausea and vomiting induced by chemotherapy, radiotherapy or surgery. Drugs 1997; 54: 273-98. [PubMed: 9257083](Review of the pharmacokinetics, efficacy and safety of dolasetron as therapy of nausea and vomiting caused by chemotherapy or surgery; liver test abnormalities have been reported, but not characterized).
- Weiss KS. Anaphylactic reaction to ondansetron. Arch Intern Med 2001; 161: 2263. [PubMed: 11575988](61 year old woman developed flushing and tongue swelling 5 minutes after an initial infusion of ondansetron, resolving with symptomatic therapy; no mention of ALT levels).
- Camilleri M, Chey WY, Mayer EA, Northcutt AR, Heath A, Dukes GE, McSorley D, et al. A randomized controlled clinical trial of the serotonin type 3 receptor antagonist alosetron in women with diarrhea-predominant irritable bowel syndrome. Arch Intern Med 2001; 161: 1733-40. [PubMed: 11485506](Among 626 patients with diarrhea predominant IBS treated with either alosetron [1 mg] or placebo twice daily for 12 weeks, constipation was the most common adverse event [25% vs 5%] and "laboratory values were not significantly affected by alosetron treatment").
- Kamm MA. Review article: the complexity of drug development for irritable bowel syndrome. Aliment Pharmacol Ther 2002; 16: 343-51. [PubMed: 11876686](History of development of serotonin modifying drugs for irritable bowel syndrome [IBS], and particularly alosetron which is 10 times more potent than ondansetron and was approved for use in diarrhea predominant IBS, but was later withdrawn because of frequent complications of constipation and several episodes of ischemic colitis, some of which were fatal).
- Cremonini F, Delgado-Aros S, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Neurogastroenterol Motil 2003; 15: 79-86. [PubMed: 12588472](Analysis of 6 controlled trials of alosetron vs placebo in IBS found no significant differences in frequency of adverse events, except for constipation, between the two groups).
- Kim JS, Baek JY, Park SR, Choi IS, Kim SI, Kim DW, Im SA, et al. Open-label, randomized comparison of the efficacy of intravenous dolasetron mesylate and ondansetron in the prevention of acute and delayed cisplatin-induced emesis in cancer patients. Cancer Res Treat 2004 36: 372-6. [PMC free article: PMC2843880] [PubMed: 20368831](Among 112 patients given ondansetron or dolasetron to prevent chemotherapy induced nausea and vomiting, there were no "clinically relevant differences found between the treatment groups with respect to laboratory test results").
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants done in the US between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, but none were attributed to antiemetic agents or the 5-HT3 receptor blockers).
- Turgeon DK, Tayeh N, Fontana RJ. Acute hepatitis associated with alosetron(Lotronex). J Clin Gastroenterol 2005; 39: 641-2. [PubMed: 16000936](39 year old woman developed malaise and nausea four weeks after starting alosetron for chronic diarrhea [peak bilirubin normal, ALT 160 U/L, Alk 342 U/L], which had returned to normal when tested 6 months later).
- Piche T, Vanbiervliet G, Cherikh F, Antoun Z, Huet PM, Gelsi E, Demarquay JF, et al. Effect of ondansetron, a 5-HT3 receptor antagonist, on fatigue in chronic hepatitis C: a randomised, double blind, placebo controlled study. Gut 2005; 54: 1169-73. [PMC free article: PMC1774898] [PubMed: 16009690](Among 36 patients with chronic hepatitis C and fatigue treated with tablets of ondansetron [4 mg] or placebo tablets twice daily for one month, there were no significant changes in ALT levels in either group).
- Theal JJ, Toosi MN, Girlan L, Heslegrave RJ, Huet PM, Burak KW, Swain M, et al. A randomized, controlled crossover trial of ondansetron in patients with primary biliary cirrhosis and fatigue. Hepatology 2005; 41: 1305-12. [PubMed: 15915460](Among 60 patients with primary biliary cirrhosis and fatigue treated with ondansetron [4 mg three times daily] or placebo for 4 days, there was no evidence of a reduction in fatigue; serum ALT levels did not change).
- O'Donohue JW, Pereira SP, Ashdown AC, Haigh CG, Wilkinson JR, Williams R. A controlled trial of ondansetron in the pruritus of cholestasis. Aliment Pharmacol Ther 2005; 21: 1041-5. [PubMed: 15813840](Among 19 patients with resistant pruritus treated with ondansetron [8 mg twice daily] or placebo for 5 days, scratching and pruritus decreased with the medication and one patient developed a rise in Alk P [306 to 812 U/L] and bilirubin [3.7 to 44.3 mg/dL], but no further details given).
- Lewandowski MJ, Chapman SA. Ondansetron-induced aminotransferase level elevation: case report and review of the literature. Pharmacotherapy 2008; 28: 1542-6. [PubMed: 19025436](44 year old woman received intravenous ondansetron on 3 occasions during emergency room evaluation for chest pain and nausea and developed ALT elevations within 12-48 hours each time [bilirubin 0.7 mg/dL, ALT 816, 798 and 761 U/L, Alk P 121 U/L], which fell to normal within a few days).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to anti emetic agents or the 5-HT3 receptor blockers).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to antiemetic agents or the 5-HT3 receptor blockers).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol 2010; 70: 721-8. [PMC free article: PMC2997312] [PubMed: 21039766](Among 624,673 adverse event reports in children between 2000 and 2006 in the WHO VigiBase, 1% were hepatic, but no 5-HT3 receptor blocker was listed among the 41 most commonly implicated agents).
- Vergne-Salle P, Dufauret-Lombard C, Bonnet C, Simon A, Trèves R, Bonnabau H, Bertin P. A randomised, double-blind, placebo-controlled trial of dolasetron, a 5-hydroxytryptamine 3 receptor antagonist, in patients with fibromyalgia. Eur J Pain 2011; 15: 509-14. [PubMed: 21036635](Among 60 patients with fibromyalgia treated with 4 monthly infusions of dolasetron or placebo, there were "no meaningful changes" in ALT, AST or Alk P levels; one patient on dolasetron developed rise in ALT to 4 times ULN that resolved within 3 weeks).
- Schwartzberg L, Barbour SY, Morrow GR, Ballinari G, Thorn MD, Cox D. Pooled analysis of phase III clinical studies of palonosetron versus ondansetron, dolasetron, and granisetron in the prevention of chemotherapy-induced nausea and vomiting(CINV). Support Care Cancer 2014; 22: 469-77. [PMC free article: PMC3889920] [PubMed: 24141698](Analysis of 4 controlled trials comparing palonosetron to ondansetron, dolasetron and granisetron for prevention of nausea and vomiting after cancer chemotherapy found similar rates of adverse events 20-27%; ALT elevations in 0.2% and 2.1% of patients on palonosetron and 3.1% on other 5-HT3 receptor antagonists).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to antiemetics or 5-HT3 receptor blockers).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, the most common implicated agents being nimesulide [n=53: 30%], cyproterone [n=18], nitrofurantoin [n=17], antituberculosis drugs [n=13] and flutamide [n=12: 7%]; antiemetics and metoclopramide were not listed).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to a serotonin 5-HT3 receptor antagonist).
- Simino GP, Marra LP, Andrade EI, Acúrcio Fde A, Reis IA, De Araújo VE, Cherchiglia ML. Efficacy, safety and effectiveness of ondansetron compared to other serotonin-3 receptor antagonists (5-HT3RAs) used to control chemotherapy-induced nausea and vomiting: systematic review and meta-analysis. Expert Rev Clin Pharmacol 2016; 9: 1183-94. [PubMed: 27180992](Review of the literature on the safety and efficacy of the 5-HT3 receptor blockers used as antiemetics, discusses symptoms of headache, dizziness, constipation and diarrhea, but not ALT or other laboratory abnormalities; no mention of hepatotoxicity).
- Park H, Oh K, Lee H, Lee JH, Kang SM, Park SY, Kwon HS, Cho YS, Moon HB, Kim TB. Palonosetron-induced anaphylaxis during general anesthesia: A case report. Allergy Asthma Immunol Res 2017; 9: 92-5. [PMC free article: PMC5102841] [PubMed: 27826967](37 year old man developed hypotension immediately after an infusion of palonosetron during surgery with subsequent rash and facial edema, responding to corticosteroid therapy; no mention of ALT elevations or hepatotoxicity).
- Kovács G, Wachtel A, Basharova E, Spinelli T, Nicolas P, Kabickova E. Palonosetron compared with ondansetron in pediatric cancer patients: multicycle analysis of a randomized Phase III study. Future Oncol 2017; 13: 1685-98. [PubMed: 28569078](Among 502 children receiving up to 4 cycles of cancer chemotherapy who were treated with one of two doses of palonosetron or ondansetron, higher doses of palonosetron were associated with fewer emetic episodes while adverse event rates were similar; no mention of ALT elevations or hepatotoxicity).
- Takahashi T, Okada T, Ikejiri F, Ito S, Okada Y, Takahashi F, Kumanomido S, et al. A prospective study of palonosetron for prevention of chemotherapy-induced nausea and vomiting in malignant lymphoma patients following highly emetogenic chemotherapy. Int J Clin Oncol 2017 Aug 19. [PubMed: 28823027](Among 59 patients with lymphoma treated with palonosetron, 94% had no nausea or vomiting and adverse events were mild including ALT elevations in 6 [12%], all of which were less than 5 times ULN).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review 5-HT3 receptor antagonists for the prevention of chemotherapy-induced nausea and vomiting. A comparison of their pharmacology and clinical efficacy.[Drugs. 1998]Review 5-HT3 receptor antagonists for the prevention of chemotherapy-induced nausea and vomiting. A comparison of their pharmacology and clinical efficacy.Gregory RE, Ettinger DS. Drugs. 1998 Feb; 55(2):173-89.
- Review Spectrum of use and tolerability of 5-HT3 receptor antagonists.[Scand J Rheumatol Suppl. 2004]Review Spectrum of use and tolerability of 5-HT3 receptor antagonists.Haus U, Späth M, Färber L. Scand J Rheumatol Suppl. 2004; 119:12-8.
- Review Comparative Pharmacology and Guide to the Use of the Serotonin 5-HT(3) Receptor Antagonists for Postoperative Nausea and Vomiting.[Drugs. 2016]Review Comparative Pharmacology and Guide to the Use of the Serotonin 5-HT(3) Receptor Antagonists for Postoperative Nausea and Vomiting.Kovac AL. Drugs. 2016 Dec; 76(18):1719-1735.
- Review Use of dexamethasone with 5-HT3-receptor antagonists for chemotherapy-induced nausea and vomiting.[Cancer J Sci Am. 1998]Review Use of dexamethasone with 5-HT3-receptor antagonists for chemotherapy-induced nausea and vomiting.Perez EA. Cancer J Sci Am. 1998 Mar-Apr; 4(2):72-7.
- 5-HT3 receptors as important mediators of nausea and vomiting due to chemotherapy.[Biochim Biophys Acta. 2015]5-HT3 receptors as important mediators of nausea and vomiting due to chemotherapy.Navari RM. Biochim Biophys Acta. 2015 Oct; 1848(10 Pt B):2738-46. Epub 2015 Mar 30.
- Serotonin 5-HT3 Receptor Antagonists - LiverToxSerotonin 5-HT3 Receptor Antagonists - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...