NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Selumetinib is an oral, small molecule inhibitor of the mitogen activated protein kinase 1 and 2 (MEK1/2) that is used to treat symptomatic, refractory fibromas in neurofibromatosis type 1. Selumetinib is associated with transient and usually mild elevations in serum aminotransferase levels during therapy, but has not been linked to cases of clinically apparent acute liver injury.
Background
Selumetinib (sel" ue me’ ti nib) is an orally available, specific inhibitor of mitogen activated protein kinase 1 and 2 (MEK1/2) and is used to treat neurofibromatosis. Neurofibromatosis type 1 is marked by a mutation in the neurofibromin, a tumor suppressor gene which is a negative regulator of the RAS/MAPK signaling pathway. The mutant neurofibromin causes dysregulation of the RAF/MEK/ERK pathway and promotes tumor growth. In open label trials, selumetinib was found to improve symptoms and result in decrease in volume and distribution of neurofibromas in children with neurofibromatosis type 1. While partial responses occurred in more than half of patients, none had a complete response and resolution of all neurofibromas. Selumetinib was approved for use in neurofibromatosis in the United States in 2020. It is also being evaluated in other forms of cancer, such as melanoma, gliomas, and non-small cell lung cancer in which MEK1/2 is overexpressed. Current indications are for children above the age of 2 and adults with neurofibromatosis type 1 with symptomatic, inoperable plexiform neurofibromas. It is available in capsules of 10 and 25 mg under the brand name Koselugo. The usual recommended dose 25 mg/m2 twice daily. Side effects are very common and include rash, diarrhea, nausea, vomiting, abdominal pain, fatigue, fever, stomatitis, headache, paronychia and pruritus. Severe adverse events occur in approximately 24% of patients and lead to discontinuation in 12%. Severe adverse events include cardiomyopathy, ocular abnormalities with blurred and decreased vision, severe diarrhea and dehydration, severe skin rash, marked CPK elevations and rarely rhabdomyolysis, and embryo-fetal toxicity. Both cardiac and ophthalmologic monitoring is recommended.
Hepatotoxicity
In the prelicensure clinical trials conducted in children and adults with neurofibromatosis, serum aminotransferase elevations occurred in 35% of treated subject but rose to above 5 times the upper limit of normal (ULN) in only 4%. However, there were no liver related serious adverse events and no patient had a concurrent elevation in serum aminotransferase and bilirubin levels. The ALT elevations were typically mild and transient and usually resolved even without dose adjustment. There were no instances of clinically apparent liver injury attributed to selumetinib. Since approval and more wide scale availability of selumetinib, there have been no published reports of clinically apparent drug induced liver injury associated with its use in neurofibromatosis, although clinical experience with the drug, particularly with long term therapy, has been limited. However, in studies of experimental therapy with somewhat higher doses of selumetinib in patients with advanced, refractory cancers, liver test abnormalities were common and sometimes graded as severe (ALT above 20 times ULN) and requiring drug discontinuation. Thus, in patients with neurofibromatosis and use of recommended doses of selumetinib, clinically apparent liver injury is rare if it occurs at all. At higher doses, however, selumetinib has been associated with a very high rate of serum enzyme elevations, many of which were in the range suggestive of severe injury.
Likelihood score: E* (suspected but unproven rare cause of clinically apparent liver injury).
Mechanism of Injury
The causes of serum enzyme elevations during selumetinib therapy are not known. Selumetinib is metabolized in the liver largely through the cytochrome P450 pathway and specifically by CYP 3A4, and liver injury may be related to production of a toxic or immunogenic intermediate. Because it is a substrate for CYP 3A4, selumetinib is susceptible to drug-drug interactions with agents that inhibit or induce this specific hepatic microsomal activity.
Outcome and Management
Monitoring of laboratory tests (particularly CPK) is recommended for patients treated with selumetinib. Serum aminotransferase elevations above 5 times the upper limit of normal (if confirmed) or any elevations accompanied by jaundice or symptoms should lead to dose reduction or temporary cessation. There are no data to suggest a cross reactivity in risk for hepatic injury between selumetinib and other kinase inhibitors.
Drug Class: Genetic Disorder Agents, Antineoplastic Agents, Protein Kinase Inhibitors
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Selumetinib – Koselugo®
DRUG CLASS
Genetic Disorder Agents, Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
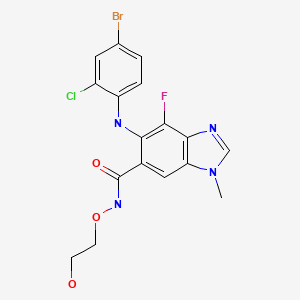
| Selumetinib | 606143-52-6 | C17-H15-Br-Cl-F-N4-O3 |
|
ANNOTATED BIBLIOGRAPHY
References updated: January 21, 2021
- Zimmerman HJ. Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of protein kinase inhibitors such as selumetinib).
- DeLeve LD. Erlotinib. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 556.(Review of hepatotoxicity of cancer chemotherapeutic agents discusses several kinase inhibitors including imatinib, gefitinib, erlotinib and crizotinib, but not selumetinib).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Pathway-targeted therapies: monoclonal antibodies, protein kinase inhibitors, and various small molecules. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1203-36.(Textbook of pharmacology and therapeutics; selumetinib is not discussed specifically).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2020/213756Orig1s000MultidisciplineR.pdf. (FDA website with medical review of efficacy and safety of selumetinib which formed the basis of its approval for use in neurofibromatosis, mentions that in registration trials in adults and children with neurofibromatosis there were no liver related serious adverse events, and although ALT elevations arose in 35% of children, none were associated with symptoms or jaundice). - Shah RR, Morganroth J, Shah DR. Hepatotoxicity of tyrosine kinase inhibitors: clinical and regulatory perspectives. Drug Saf. 2013;36:491–503. [PubMed: 23620168](Review of the hepatotoxicity of 18 tyrosine kinase inhibitors approved for use in cancer in the US as of 2013; selumetinib is not discussed).
- Spraggs CF, Xu CF, Hunt CM. Genetic characterization to improve interpretation and clinical management of hepatotoxicity caused by tyrosine kinase inhibitors. Pharmacogenomics. 2013;14:541–54. [PubMed: 23556451](Review of genetic associations of serum ALT and bilirubin elevations during therapy with tyrosine kinase inhibitors focusing on lapatinib and pazopanib; selumetinib is not mentioned).
- Dombi E, Baldwin A, Marcus LJ, Fisher MJ, Weiss B, Kim A, Whitcomb P, et al. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med. 2016;375:2550–60. [PMC free article: PMC5508592] [PubMed: 28029918](Among 24 children with neurofibromatosis and symptomatic plexiform neurofibromas treated in 28 day cycles of 3 doses of selumetinib [20, 25 or 30 mg/m2 twice daily] for a median of 30 cycles, tumor volume decreased in all subjects and dose limiting adverse events included CPK elevations, rash, cellulitis, mucositis and decline in ventricular ejection fraction; 11 episodes of ALT elevation arose but all were transient and mild [less than 3 times ULN]).
- Holkova B, Zingone A, Kmieciak M, Bose P, Badros AZ, Voorhees PM, Baz R, et al. A phase II trial of AZD6244 (selumetinib, ARRY-142886), an oral MEK1/2 inhibitor, in relapsed/refractory multiple myeloma. Clin Cancer Res. 2016;22:1067–75. [PMC free article: PMC4775365] [PubMed: 26446942](Among 36 patients with refractory or relapsed multiple myeloma treated with selumetinib [75 mg twice daily in 28 day cycles], only 2 [6%] had an objective response and adverse events were common, mostly hematologic, 2 patients developing ALT elevations, one of which was above 20 times ULN).
- Alves Júnior SF, Zanetti G, Alves de Melo AS, Souza AS Jr, Souza LS, de Souza Portes Meirelles G, Irion KL, et al. Neurofibromatosis type 1: State-of-the-art review with emphasis on pulmonary involvement. Respir Med. 2019;149:9–15. [PubMed: 30885426](Review of the pathogenesis, epidemiology, clinical features and natural history of neurofibromatosis type 1 [von Recklinghausen disease], an autosomal dominant condition affecting 1:3000 persons that typically presents in childhood with café au lait spots, multiple neurofibromas, pigmented hamartomas, in the iris, seizures, bone and eye disease and, in adulthood, diffuse lung disease).
- Fangusaro J, Onar-Thomas A, Young Poussaint T, Wu S, Ligon AH, Lindeman N, Banerjee A, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 2019;20:1011–22. [PMC free article: PMC6628202] [PubMed: 31151904](Among 50 children with low grade gliomas treated with selumetinib [25 mg/m2 twice daily], partial responses occurred in 35-40% and adverse events were frequent, ALT elevations arising in 21 [42%], but all but one was mild [less than 3 times ULN] and none were associated with symptoms or jaundice or required drug discontinuation).
- Gross AM, Wolters PL, Dombi E, Baldwin A, Whitcomb P, Fisher MJ, Weiss B, et al. Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med. 2020;382:1430–42. [PMC free article: PMC7305659] [PubMed: 32187457](Among 50 children with neurofibromatosis and symptomatic plexiform neurofibromas treated with selumetinib in 28 day cycles, tumor volume decreased in 34 [68%] which was durable in 28 [56%] and accompanied by improvements in pain scores, quality of life and strength; adverse events were common and led to discontinuation in 10%; ALT elevations arose in 14 [28%], but only 1 [2%] was above 3 times ULN).
- Markham A, Keam SJ. Selumetinib: first approval. Drugs. 2020;80:931–7. [PubMed: 32504375](Review of the history of development, pharmacology, mechanism of action, clinical efficacy and safety of selumetinib shortly after its initial approval as therapy of neurofibromatosis type 1 in the US; mentions occurrence of ALT elevations in 35% of subjects, but does not mention episodes of clinically apparent hepatotoxicity).
- Kenney C, Kunst T, Webb S, Christina D Jr, Arrowood C, Steinberg SM, Mettu NB, et al. Phase II study of selumetinib, an orally active inhibitor of MEK1 and MEK2 kinases, in KRAS-G12R-mutant pancreatic ductal adenocarcinoma. Invest New Drugs. 2021 Jan 6; Epub ahead of print. [PMC free article: PMC8068685] [PubMed: 33405090](Among 8 patients with advanced pancreatic cancer and KRAS variants treated with selumetinib [75 mg twice daily], none had even a partial objective response and side effects were common, all 8 developed ALT elevations which were above 20 times ULN in 2 requiring dose discontinuation or reduction, one subject also developed jaundice; few details given).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Selumetinib: First Approval.[Drugs. 2020]Review Selumetinib: First Approval.Markham A, Keam SJ. Drugs. 2020 Jun; 80(9):931-937.
- Review A Review of Selumetinib in the Treatment of Neurofibromatosis Type 1-Related Plexiform Neurofibromas.[Ann Pharmacother. 2022]Review A Review of Selumetinib in the Treatment of Neurofibromatosis Type 1-Related Plexiform Neurofibromas.Anderson MK, Johnson M, Thornburg L, Halford Z. Ann Pharmacother. 2022 Jun; 56(6):716-726. Epub 2021 Sep 18.
- Activity of Selumetinib in Neurofibromatosis Type 1-Related Plexiform Neurofibromas.[N Engl J Med. 2016]Activity of Selumetinib in Neurofibromatosis Type 1-Related Plexiform Neurofibromas.Dombi E, Baldwin A, Marcus LJ, Fisher MJ, Weiss B, Kim A, Whitcomb P, Martin S, Aschbacher-Smith LE, Rizvi TA, et al. N Engl J Med. 2016 Dec 29; 375(26):2550-2560.
- Selumetinib side effects in children treated for plexiform neurofibromas: first case reports of peripheral edema and hair color change.[BMC Pediatr. 2021]Selumetinib side effects in children treated for plexiform neurofibromas: first case reports of peripheral edema and hair color change.Baldo F, Magnolato A, Barbi E, Bruno I. BMC Pediatr. 2021 Feb 6; 21(1):67. Epub 2021 Feb 6.
- Population pharmacokinetics and exposure-response of selumetinib and its N-desmethyl metabolite in pediatric patients with neurofibromatosis type 1 and inoperable plexiform neurofibromas.[Cancer Chemother Pharmacol. 2021]Population pharmacokinetics and exposure-response of selumetinib and its N-desmethyl metabolite in pediatric patients with neurofibromatosis type 1 and inoperable plexiform neurofibromas.Schalkwijk S, Zhou L, Cohen-Rabbie S, Jain L, Freshwater T, So K, He Z, Gioni I, Tomkinson H, Vishwanathan K, et al. Cancer Chemother Pharmacol. 2021 Aug; 88(2):189-202. Epub 2021 Apr 26.
- Selumetinib - LiverToxSelumetinib - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...