NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Streptozocin is a unique antineoplastic agent used to treat metastatic pancreatic islet cell carcinoma. Streptozocin has been associated with a high rate of serum enzyme elevation during therapy and to rare instances of severe, clinically apparent acute liver injury.
Background
Streptozocin (strep" toe zoe' sin), which was formerly known as streptotozocin, is an antineoplastic antibiotic isolated initially from Streptomyces achromogenes. Structurally, streptozocin is a methylnitrosourea attached to a single molecule of glucosamine and acts as an alkylating agent causing DNA damage and interfering with synthesis. Streptozocin has high affinity for the cell surface glucose transporter GLUT 2 which is highly expressed on beta cells of the islets of Langerhans. As a consequence, streptozocin has differential toxicity to beta cells. In animal models, streptozocin caused loss of pancreatic islet cells, resulting in a decrease in serum insulin and diabetes. Used initially as a means of creating an animal model of type 1 diabetes, streptozocin was later used clinically to treat insulin-secreting islet cell tumors. It was tried, but had little effect in other malignancies. Streptozocin was approved for use in the chemotherapy of metastatic pancreatic islet cell carcinoma in the United States in 1982 and is still used, but largely in combination with other chemotherapeutic agents such as doxorubicin and fluorouracil. In addition, it has been used off label to treat other neuroendocrine neoplasms. Streptozocin is available as a powder for reconstitution in 1 gram vials under the brand name Zanosar. The typical dose is 500 mg/m2 daily for 5 days every 6 weeks or as 1 gram once weekly intravenously until a remission is achieved or significant toxicity intervenes. Common side effects of streptozocin therapy are nausea, vomiting, diarrhea, abdominal discomfort and local infusion site reactions. These immediate reactions can be followed by renal dysfunction (proteinuria, proximal tubular injury, phosphaturia, acute renal failure), bone marrow suppression (anemia, neutropenia) and hepatotoxicity.
Hepatotoxicity
Serum aminotransferase elevations occur in up to two-thirds of patients treated with streptozocin, but the abnormalities are generally mild, transient and not associated with symptoms or jaundice. Hepatotoxicity is more common with daily dosing and high doses of streptozocin, but with higher doses renal and hematologic toxicities usually overshadow hepatic injury. There have been two reports of rapidly progressive and fatal acute liver failure in patients treated with streptozocin. In one instance, no other chemotherapy was given, in another fluorouracil was coadministered and the patient presented with fever, anuria, acute hepatitis [ALT 1280, bilirubin 11.9, prothrombin index 10%, eosinophils 2600/ µL] at the end of a 5 day course of treatment. In contrast, there have been no individual published case reports of self-limited clinically apparent liver injury attributed to streptozocin, but it has had limited use, as pancreatic islet cell carcinoma and neuroendocrine tumors are rare.
Likelihood score: D (possible cause of clinically apparent liver injury).
Mechanism of Injury
Like the renal and bone marrow toxicity, the hepatic injury from streptozocin is likely due to a direct effect of the alkylating agent and its limited uptake by hepatocytes. Higher doses have been shown to cause liver injury in experimental animals.
Outcome and Management
The severity of the liver injury linked to streptozocin therapy has been generally mild, transient and without symptoms or jaundice. Streptozocin has not been linked to cases of chronic hepatitis or vanishing bile duct syndrome. There is no information on cross sensitivity to hepatic injury between streptozocin and other antineoplastic agents.
Drug Class: Antineoplastic Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Streptozocin – Zanosar®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
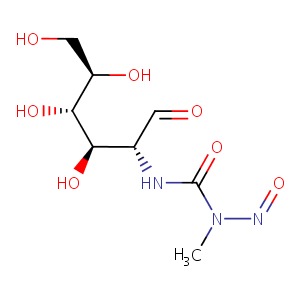
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Streptozocin | 18883-66-4 | C8-H15-N3-O7 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 23 August 2016
- Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999; mentions that streptozocin can cause elevations in serum aminotransferase levels "but the abnormalities are of little clinical moment").
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam, Elsevier, 2013, p. 541-68.(Review of hepatotoxicity of cancer chemotherapeutic agents; streptozocin is listed as causing transient liver test abnormalities, but not clinically apparent liver injury).
- Chabner BA, Bertino J, Cleary J, Ortiz T, Lane A, Supko JG, Ryan DP. Cytotoxic agents. Chemotherapy of neoplastic diseases. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, p. 1698.(Textbook of pharmacology and therapeutics).
- Junod A, Lambert AE, Orci L, Pictet R, Gonet AE, Renold AE. Studies of the diabetogenic action of streptozotocin. Proc Soc Exp Biol Med 1967; 126: 201-5. [PubMed: 4864021](Studies in rats using purified streptozocin demonstrated a highly specific pancreatic beta cell injury with subsequent decrease in serum insulin levels and development of type 1 diabetes; the authors propose its use to develop animal models of diabetes and as therapy of insulin secreting islet cell tumors).
- Murray-Lyon IM, Eddleston AL, Williams R, Brown M, Hogbin BM, Bennett A, Edwards JC, Taylor KW. Treatment of multiple-hormone-producing malignant islet-cell tumour with streptozotocin. Lancet 1968; 2 (7574): 895-8. [PubMed: 4176152](59 year old woman with metastatic, insulin producing islet cell cancer was treated with 3 infusions of streptozocin and achieved a long term remission).
- Taylor SG 3rd, Schwartz TB, Zannini JJ, Ryan WG. Streptozotocin therapy for metastatic insulinoma. Arch Intern Med 1970; 126: 654-7. [PubMed: 4319181](70 year old man with metastatic, insulin-producing pancreatic islet cell tumor had a complete remission and correction of hypoglycemia after 5 infusions of streptozocin over a 7 week period).
- Sadoff L. Nephrotoxicity of streptozotocin (NSC-85998). Cancer Chemother Rep 1970; 54 (6): 457-9. [PubMed: 4334090](Among 18 patients with various malignancies treated with intravenous streptozocin [2 grams/m2 once weekly], all had some degree of renal tubular toxicity with hypophosphatemia in 14 [5 developing osteomalacia within 6 months], glycosuria in 14, renal tubular acidosis in 10, azotemia in 9, and anuria in 2, one of whom died; 9 patients had AST elevations and 8 hypoalbuminemia "presumably due to liver toxicity").
- Broder LE, Carter SK. Pancreatic islet cell carcinoma. II. Results of therapy with streptozotocin in 52 patients. Ann Intern Med 1973; 79: 108-18. [PubMed: 4352784](Summary of experience using streptozocin for pancreatic islet cell tumors in 52 patients participating in an NCI registry reported a 60% response rate, but toxicities included 7 deaths, 5 from acute renal failure but none from liver injury, despite liver test abnormalities arising in 67% of cases [ALT 16%, AST 27%, Alk P 12%, and bilirubin 4%]; but "hepatic toxicity was difficult to evaluate because most patients had liver metastases").
- Schein P, Kahn R, Gorden P, Wells S, Devita VT. Streptozotocin for malignant insulinomas and carcinoid tumor. Report of eight cases and review of the literature. Arch Intern Med 1973; 132: 555-61. [PubMed: 4355081](Among 8 patients with malignant insulinomas or carcinoid syndrome treated with streptozocin at the NIH Clinical Center, responses occurred only in islet cell tumors and 6 patients had renal toxicity [proteinuria, phosphaturia] which was reversible; and 5 had transient and asymptomatic AST elevations [63-122 U/L]).
- Schein PS, O'Connell MJ, Blom J, Hubbard S, Magrath IT, Bergevin P, Wiernik PH, Ziegler JL, DeVita VT. Clinical antitumor activity and toxicity of streptozotocin (NSC-85998). Cancer 1974; 34: 993-1000. [PubMed: 4371075](Among 106 patients with advanced malignancies given streptozocin, responses were not common and limited to lymphomas and islet cell cancer and toxicity included nausea and vomiting [87%] poorly controlled by antiemetics, renal toxicity [28%], and liver enzyme elevations [15%] appearing 1-2 days after the course and rapidly returning to normal without jaundice or symptoms).
- Loftus L, Cuppage FE, Hoogstraten B. Clinical and pathological effects of streptozotocin. J Lab Clin Med 1974; 84: 407-13. [PubMed: 4368506](One patient with adenocarcinoma and another with islet cell cancer received multiple courses of streptozocin, one dying of renal failure and the second of progressive disease, both having cellular atypia in the liver on autopsies).
- Herbai G, Lundin A. Treatment of malignant metastatic pancreatic insulinoma with streptozotocin. Review of 21 cases described in detail in the literature and report of complete remission of a new case. Acta Med Scand 1976; 200: 447-52. [PubMed: 189576](55 year old woman with metastatic, insulin secreting islet cell cancer had a remission after 7 infusions of streptozocin; "liver enzyme values and kidney function revealed no abnormalities").
- Hayes JR, O'Connell N, O'Neill T, Fennelly JJ, Weir DG. Successful treatment of a malignant gastrinoma with streptozotocin. Gut 1976; 17: 285-8. [PMC free article: PMC1411094] [PubMed: 178577](36 year old woman with gastrin producing islet cell tumor did not respond to streptozocin given intravenously, but did after infusions in the celiac artery during which there was a 3 fold increase in ALT).
- Meyer DJ. Temporary remission of hypoglycemia in a dog with an insulinoma after treatment with streptozotocin. Am J Vet Res 1977; 38: 1201-4. [PubMed: 199092](7 year old male dog with metastatic insulin secreting pancreatic islet cell tumor developed vomiting followed by proteinuria and liver test abnormalities after a second dose of streptozocin which recurred with another course of treatment [peak ALT 310 and 420 U/L], liver histology at autopsy showing cancer, but no injury to normal liver tissue).
- Oberg K, Boström H, Fahrenkrug J, Dymling JF, Shaffalitsky de Muckadell OB, Lundqvist G. Streptozotocin treatment of a pancreatic tumour producing VIP and gastrin associated with Verner-Morrison syndrome. Acta Med Scand 1979; 206: 223-7. [PubMed: 227234](57 year old man with metastatic, gastrin and VIP secreting pancreatic islet cell cancer had a long term remission after 3 weekly infusions of streptozocin [2 g each]; no mention of side effects).
- Moertel CG, Hanley JA, Johnson LA. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med 1980; 303: 1189-94. [PubMed: 6252466](Among 84 patients with islet cell cancers treated with streptozocin alone or with fluorouracil, response rates were higher with the combination [63% vs 36%] as was survival, while adverse events included marked nausea and vomiting [85% vs 83%], nephrotoxicity [31% vs 29%] and leukopenia [73% vs 5%] and one patient given streptozocin alone developed acute liver failure immediately after therapy and died on day 18, but no further details provided).
- Weiss RB. Streptozocin: a review of its pharmacology, efficacy, and toxicity. Cancer Treat Rep 1982; 66: 427-38. [PubMed: 6277485](Review of the chemistry, pharmacology, efficacy and toxicity of streptozocin mentions that chemical liver dysfunction occurs in 25% of patients, usually 1-2 days after an intravenous course, but the injury is only occasionally severe).
- Luquel L, Ekherian JM, De Gramont A, Offenstadt G. [Anuria, hepatocellular insufficiency and bone marrow aplasia after the administration of streptozocin and fluorouracil]. Therapie 1988; 43: 125. French. [PubMed: 2970126](52 year old woman developed fever and anuria between days 4 and 6 of a third 5-day course of streptozocin and fluorouracil with rapidly progressive renal, hematologic and liver failure [bilirubin 11.9 mg/dL, ALT 1280, prothrombin index 10%, eosinophils 2600/µL], resulting in death within 8 days; no autopsy performed).
- Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med 1992; 326: 519-23. [PubMed: 1310159](Among 105 patients with pancreatic islet cell cancer treated with various chemotherapy regimens, streptozocin with doxorubicin yielded the highest rate of tumor regression [69%] and survival rates, while toxicities included severe nausea and vomiting during treatment and delayed renal and hematologic toxicities including one death from sepsis; no mention of ALT elevations or hepatotoxicity).
- Kume E, Fujimura H, Matsuki N, Ito M, Aruga C, Toriumi W, Kitamura K, Doi K. Hepatic changes in the acute phase of streptozotocin (SZ)-induced diabetes in mice. Exp Toxicol Pathol 2004; 55: 467-80. [PubMed: 15384252](Study of the direct, acute hepatic toxicity of streptozocin in mice that showed fat droplet accumulation within 24 hours of infusions).
- Aoki T, Kokudo N, Komoto I, Takaori K, Kimura W, Sano K, Takamoto T, et al. Streptozocin chemotherapy for advanced/metastatic well-differentiated neuroendocrine tumors: an analysis of a multi-center survey in Japan. J Gastroenterol 2015; 50: 769-75. [PMC free article: PMC4493796] [PubMed: 25348496](Among 54 patients with neuroendocrine tumors treated with streptozocin at 5 Japanese cancer centers, 13 [24%] had a partial or complete response and "the adverse event profile was mild and tolerable," with mild liver test abnormalities in only one patient).
- Krug S, Boch M, Daniel H, Nimphius W, Müller D, Michl P, Rinke A, et al. Streptozocin-Based Chemotherapy in Patients with Advanced Neuroendocrine Neoplasms--Predictive and Prognostic Markers for Treatment Stratification. PLoS One 2015; 10 (12): e0143822. [PMC free article: PMC4668106] [PubMed: 26630134](Among 77 patients with advanced neuroendocrine tumors seen at a single German referral center between 1995-2013 who were treated with streptozocin with either doxorubicin or fluorouracil [5-FU], the objective response rate was 34% and toxicities were common with serum enzyme elevations in 65% of subjects, which were above 5 times ULN in 5%).
- Clewemar Antonodimitrakis P, Sundin A, Wassberg C, Granberg D, Skogseid B, Eriksson B. Streptozocin and 5-Fluorouracil for the Treatment of Pancreatic Neuroendocrine Tumors: Efficacy, Prognostic Factors and Toxicity. Neuroendocrinology 2016; 103 (3-4): 345-53. [PubMed: 26279284](Among 137 patients with neuroendocrine tumors treated with streptozocin and fluorouracil [5-FU] at a Swedish cancer center between 1981 and 2014, 3% had a complete and 25% a partial response while toxicities were "acceptable"; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Doxorubicin, streptozocin, and 5-fluorouracil chemotherapy for patients with metastatic islet-cell carcinoma.[Am J Clin Oncol. 1998]Doxorubicin, streptozocin, and 5-fluorouracil chemotherapy for patients with metastatic islet-cell carcinoma.Rivera E, Ajani JA. Am J Clin Oncol. 1998 Feb; 21(1):36-8.
- Streptozocin for treatment of pancreatic islet cell tumors in dogs: 17 cases (1989-1999).[J Am Vet Med Assoc. 2002]Streptozocin for treatment of pancreatic islet cell tumors in dogs: 17 cases (1989-1999).Moore AS, Nelson RW, Henry CJ, Rassnick KM, Kristal O, Ogilvie GK, Kintzer P. J Am Vet Med Assoc. 2002 Sep 15; 221(6):811-8.
- [Pancreatic islet cell carcinoma with multiple hepatic metastases successfully treated with a streptozocin/5-FU regimen--a case report].[Gan To Kagaku Ryoho. 2002][Pancreatic islet cell carcinoma with multiple hepatic metastases successfully treated with a streptozocin/5-FU regimen--a case report].Arakawa Y, Mizunuma N, Aiba K, Ito Y, Takahashi S, Irie T, Watanabe J, Tada K, Okudaira T, Seki M, et al. Gan To Kagaku Ryoho. 2002 Dec; 29(13):2561-4.
- Review Mitotane.[LiverTox: Clinical and Researc...]Review Mitotane.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Cabozantinib.[LiverTox: Clinical and Researc...]Review Cabozantinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Streptozocin - LiverToxStreptozocin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...