NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Tecovirimat is an orally available antiviral agent with activity against smallpox and other orthoviruses that is approved for use against smallpox virus (variola) infection in humans and has been used on a compassionate use basis disseminated vaccinia and monkeypox (now renamed “mpox”) infection. Tecovirimat therapy has not been linked to serum aminotransferase elevations or to instances of clinically apparent liver injury.
Background
Tecovirimat (tec’ oh vir’ i mat) is an unique antiviral agent that has activity against a wide spectrum of orthovirus infections including smallpox, disseminated vaccinia and mpox. Tecovirimat inhibits the orthopoxvirus VP37 envelope wrapping protein, which is required for its envelopment and secretion from infected cells and is highly conserved among orthopoxviruses. Tecovirimat was discovered by high throughput screening for agents that inhibited vaccinia-virus cytopathic effects in cell culture, as a part of a major effort to identify antiviral agents active against potential bioterrorism agents including smallpox virus. Tecovirimat was found to have broad activity against orthopoxviruses including vaccinia, mpox and rabbitpox virus and was protective against fatal infection with orthopoxviruses including variola in several animal models. Because human smallpox was successfully eradicated in the 1980s, it was not possible to do trials of tecovirimat therapy for human smallpox disease, and it was ultimately approved based upon the Animal Rule which allows appropriate animal models to be used to prove efficacy and estimate the optimal human dose regimen. Safety, however, requires proof that the proposed dose regimen is well tolerated without serious toxicity in healthy human volunteers or in patients with other viral infections. In prelicensure randomized controlled trials in healthy volunteers, tecovirimat was found to be well tolerated with low rates of minor adverse events of headache, nausea, vomiting and diarrhea. Tecovirimat was approved for treatment of smallpox in 2018 and is available as capsules of 200 mg for oral therapy and as a solution for intravenous administration in single dose vials of 200 mg in 20 mL (10 mg/mL) under the commercial name TPOXX. The recommended dose in adults and children weighing 40 to 120 kg is 600 mg twice daily for 14 days and three times daily administration is recommended for adults weighing more than 120 kg. For small children the dose is based upon body weight. Longer term therapy is not recommended. Currently, tecovirimat is being actively evaluated for efficacy and safety in treating patients with disseminated vaccinia infection and mpox virus infection. Tecovirimat appears to be generally well tolerated; mild adverse events may include headache, myalgia, fatigue, gastrointestinal upset, nausea and diarrhea. Rare but potentially severe adverse events include hypersensitivity reactions, rash and embryo-fetal injury.
Hepatotoxicity
In preregistration trials of the safety of tecovirimat in healthy adult volunteers, serum aminotransferase elevations were uncommon and mild, and were no more frequent with tecovirimat than with placebo. Furthermore, among patients treated with tecovirimat for other orthopoxvirus infections, there were no reported episodes of marked serum aminotransferase elevations or instances of clinically apparent liver injury. Thus, tecovirimat has not been shown to cause liver injury, but the total clinical experience with its use is limited.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The lack of liver injury from tecovirimat may be due to its minimal hepatic metabolism and relatively short duration of therapy. Whether longer term treatment with tecovirimat is also without serious adverse events remains to be seen. Tecovirimat is metabolized largely by hydrolysis and glucuronidation. It is a weak inducer of CYP 3A4 and mild inhibitor of CYP 2C8 and 2C19, but has few clinically significant drug-drug interactions.
Outcome and Management
Tecovirimat has not been linked to serum aminotransferase elevations or to instances of clinically apparent liver injury. There are no recommendations for screening for or monitoring routine liver tests before or during therapy.
Drug Class: Antiviral Agents
Other Drugs in the Subclass: Brincidofovir
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Tecovirimat – TPOXX®
DRUG CLASS
Antiviral Agents
Product labeling at DailyMed, National Library of Medicine, NIH
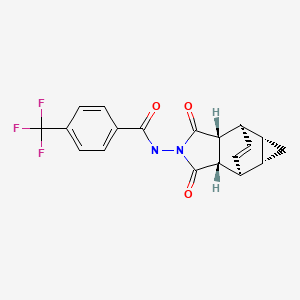
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Tecovirimat | 869572-92-9 | C19-H15-F3-N2-O3 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 18 October 2022
- Yang G, Pevear DC, Davies MH, Collett MS, Bailey T, Rippen S, Barone L, et al. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge. J Virol. 2005;79:13139–49. [PMC free article: PMC1235851] [PubMed: 16189015](Initial report of the discovery and characterization of tecovirimat [formerly ST-246] identified by high throughput screening for activity against vaccinia virus cytopathic effects in cell culture, which was shown to inhibit the highly conserved V061 [VP37] envelope wrapping protein that is required for production of extracellular orthopoxvirus and which decreased serum levels of several orthopoxviruses in infected animals, improving survival and protecting mice from orthopoxvirus induced disease).
- Jordan R, Leeds JM, Tyavanagimatt S, Hruby DE. Development of ST-246® for treatment of poxvirus infections. Viruses. 2010;2:2409–35. [PMC free article: PMC3185582] [PubMed: 21994624](Review of the development of tecovirimat, discovered by high throughput screening for inhibitors of vaccinia virus cytopathic effects in cell culture which was found to protect animals from lethal orthopoxvirus challenge, and which is orally available and well tolerated in human volunteers).
- Chinsangaram J, Honeychurch KM, Tyavanagimatt SR, Leeds JM, Bolken TC, Jones KF, Jordan R, et al. Safety and pharmacokinetics of the anti-orthopoxvirus compound ST-246 following a single daily oral dose for 14 days in human volunteers. Antimicrob Agents Chemother. 2012;56:4900–5. [PMC free article: PMC3421894] [PubMed: 22777041](Among 107 healthy volunteers treated with tecovirimat [400 or 600 mg twice daily for 14 days], therapy was safe and well tolerated with only mild nausea and headache and no change in routine laboratory test results including serum aminotransferase levels).
- Mucker EM, Goff AJ, Shamblin JD, Grosenbach DW, Damon IK, Mehal JM, Holman RC, et al. Efficacy of tecovirimat (ST-246) in nonhuman primates infected with variola virus (Smallpox). Antimicrob Agents Chemother. 2013;57:6246–53. [PMC free article: PMC3837858] [PubMed: 24100494](Among 18 cynomolgus macaques inoculated with a fatal dose of smallpox virus [variola] and treated with tecovirimat or placebo for 14 days starting on day 2 or 4, all animals were infected but those on tecovirimat had reduced levels of virus, lower total pox lesion counts [<500 vs >1000], and survived with full recovery while 3 of 6 placebo treated animals died and the other 3 had persistence of pox lesions at 28 days).
- Berhanu A, Prigge JT, Silvera PM, Honeychurch KM, Hruby DE, Grosenbach DW. Treatment with the smallpox antiviral tecovirimat (ST-246) alone or in combination with ACAM2000 vaccination is effective as a postsymptomatic therapy for monkeypox virus infection. Antimicrob Agents Chemother. 2015;59:4296–300. [PMC free article: PMC4468657] [PubMed: 25896687](Among 32 cynomolgus macaques inoculated with a fatal dose of mpox virus and treated within 3 days with smallpox vaccination [ACAM2000], tecovirimat, both or with placebo alone, all tecovirimat treated animals survived while smallpox vaccine alone had no effect; delaying tecovirimat to days 4 or 5 provided partial survival benefits and all animals that survived were protected against re-challenge).
- Hoy SM. Tecovirimat: first global approval. Drugs. 2018;78:1377–1382. [PubMed: 30120738](Review of the structure, mechanism of action, history of development, efficacy and safety of tecovirimat shortly after its approval for treatment with human smallpox infection in the US based upon its efficacy in preventing and treating mpox and rabbitpox challenge in animals and its safety in studies of its pharmacokinetics and tolerance in human volunteers).
- Russo AT, Grosenbach DW, Brasel TL, Baker RO, Cawthon AG, Reynolds E, Bailey T, et al. Effects of treatment delay on efficacy of tecovirimat following lethal aerosol monkeypox virus challenge in cynomolgus macaques. J Infect Dis. 2018;218:1490–1499. [PMC free article: PMC6151088] [PubMed: 29982575](Among 62 cynomolgus macaques inoculated with a lethal dose of mpox virus and treated with tecovirimat starting 1 to 8 days later, survival was 100% in animals started within 5 days of inoculation and was reduced with further delay [50% to 100% survival]; no mention of hepatotoxicity).
- Grosenbach DW, Honeychurch K, Rose EA, Chinsangaram J, Frimm A, Maiti B, Lovejoy C, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379:44–53. [PMC free article: PMC6086581] [PubMed: 29972742](Summary of studies of tecovirimat in animal models of orthopoxvirus infections and human trials of pharmacokinetics and safety mentions that the most common adverse events in healthy human volunteers was headache [17%] and gastrointestinal symptoms [15%] including nausea and diarrhea; no mention of ALT elevations or hepatotoxicity).
- Laudisoit A, Tepage F, Colebunders R. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379:2084–2085. [PubMed: 30462945](Letter to the editor in response to Grosenbach [2018] suggesting that tecovirimat be used to treat mpox virus infection, which occurs in several countries of Central and West Africa with more than 100 cases annually in the Republic of the Congo and with a mortality rate of 10%, its rising incidence perhaps being related in part to discontinuation of smallpox vaccination after its global eradication).
- Chan-Tack KM, Harrington PR, Choi SY, Myers L, O'Rear J, Seo S, McMillan D, et al. Assessing a drug for an eradicated human disease: US Food and Drug Administration review of tecovirimat for the treatment of smallpox. Lancet Infect Dis. 2019;19:e221–e224. [PubMed: 30853252](Summary of the basis of FDA approval of tecovirimat based upon requirements of the Animal Rule with rigorous proof of its efficacy in appropriate animal models and evidence of safety in carefully conducted studies in 359 healthy volunteers).
- Lindholm DA, Fisher RD, Montgomery JR, Davidson W, Yu PA, Yu YC, Burgado J, et al. Preemptive tecovirimat use in an active duty service member who presented with acute myeloid leukemia after smallpox vaccination. Clin Infect Dis. 2019;69:2205–2207. [PubMed: 30959520](19 year old US Airforce inductee was given smallpox vaccine and soon thereafter found to have evidence of vaccinia pox lesions and previously undiagnosed acute myelogenous leukemia, for which reasons he was given tecovirimat [600 mg twice daily for 62 days] under an expanded assessment program, which he tolerated without obvious adverse reactions attributable to tecovirimat; no mention of ALT elevations or hepatotoxicity).
- Russo AT, Berhanu A, Bigger CB, Prigge J, Silvera PM, Grosenbach DW, Hruby D. Co-administration of tecovirimat and ACAM2000™ in non-human primates: Effect of tecovirimat treatment on ACAM2000 immunogenicity and efficacy versus lethal monkeypox virus challenge. Vaccine. 2020;38:644–654. [PMC free article: PMC6954297] [PubMed: 31677948](In studies of mpox virus infection in macaques, the coadministration of tecovirimat with a live attenuated smallpox vaccine was associated with a reduced lesion response to the vaccine and partial reduction in efficacy of the vaccine, suggesting that tecovirimat by inhibiting the attenuated vaccinia virus infection caused by vaccination may also attenuate its efficacy).
- Rao AK, Schulte J, Chen TH, Hughes CM, Davidson W, Neff JM, Markarian M, et al. July 2021 Monkeypox Response Team. Monkeypox in a traveler returning from Nigeria – Dallas, Texas, July 2021. MMWR Morb Mortal Wkly Rep. 2022;71:509–516. [PMC free article: PMC8989376] [PubMed: 35389974](Case report of a middle aged man who developed fever, fatigue, cough and rash during travel in Nigeria and who presented with an extensive pustular rash 4 days after return to the United States where a West African clade of mpox virus was identified from a swab of a lesion; this being the first imported case of mpox virus infection in the US; follow up found no secondary cases in contacts).
- Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, Osborne JC, et al. NHS England High Consequence Infectious Diseases (Airborne) Network. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22:1153–1162. [PMC free article: PMC9300470] [PubMed: 35623380](Among 7 patients diagnosed with mpox disease in the UK between 2018 and 2021, 3 were treated with brincidofovir [200 mg given as 3 weekly doses], all of whom developed ALT elevations [331, 550 and 127 U/L, without jaundice] and did not complete therapy, while 1 patient received tecovirimat [600 mg twice daily for 2 weeks], with rapid resolution of rash and clearance of virus; all patients ultimately recovered).
- Matias WR, Koshy JM, Nagami EH, Kovac V, Moeng LR, Shenoy ES, Hooper DC, et al. Tecovirimat for the treatment of human monkeypox: an initial series from Massachusetts, United States. Open Forum Infect Dis. 2022;9:ofac377. [PMC free article: PMC9356679] [PubMed: 35949403](Among 3 patients with mpox virus infection presenting in Boston in Spring 2022 who were treated with tecovirimat [600 mg twice daily for 14 days], all had clinical improvements within 2-4 days and ultimately recovered; monitoring of biochemical tests found mild ALT elevations in one patient [peak 72 U/L] that resolved even without discontinuation or dose adjustment).
- O'Shea J, Filardo TD, Morris SB, Weiser J, Petersen B, Brooks JT. Interim guidance for prevention and treatment of monkeypox in persons with HIV infection – United States, August 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1023–1028. [PMC free article: PMC9400540] [PubMed: 35951495](Guidance for prevention and treatment of mpox virus infections in persons with HIV infection from the Centers for Disease Control and Prevention [CDC] in response to the multinational outbreak of mpox virus infections in May 2022; discussion of tecovirimat does not mention ALT elevations or hepatotoxicity).
- Sherwat A, Brooks JT, Birnkrant D, Kim P. Tecovirimat and the treatment of monkeypox – past, present, and future considerations. N Engl J Med. 2022;387:579–581. [PubMed: 35921403](Editorial from the FDA, NIH and CDC on the need for rapidly initiated randomized controlled trials of tecovirimat for human mpox virus infection).
- Rizk JG, Lippi G, Henry BM, Forthal DN, Rizk Y. Prevention and treatment of monkeypox. Drugs. 2022;82:957–963. [PMC free article: PMC9244487] [PubMed: 35763248](Review of the prevention and treatment of mpox virus infection mentions 2 live virus vaccines against smallpox [JYNNEOS and ACAM2000] that are approximately 85% effective against mpox; and discusses 4 antiviral agents [tecovirimat, cidofovir, brincidofovir, and vaccinia immune globulin] which are not formally approved for use in mpox can be obtained under IND or emergency access programs; mentions that brincidofovir can cause serum aminotransferase and bilirubin elevations).
- Siegrist EA, Sassine J. Antivirals with activity against monkeypox: a clinically oriented review. Clin Infect Dis. 2022 Jul 29:ciac622. Epub ahead of print. [PMC free article: PMC9825831] [PubMed: 35904001](Review of the efficacy and safety of three drugs with activity against mpox virus infection that are available in the US [cidofovir, brincidofovir and tecovirimat] mentions the superior activity of tecovirimat, the major adverse drug reactions of which being headache, abdominal pain, nausea, vomiting, dry mouth and hypersensitivity reactions).
- Lucar J, Roberts A, Saardi KM, Yee R, Siegel MO, Palmore TN. Monkeypox virus-associated severe proctitis treated with oral tecovirimat: a report of two cases. Ann Intern Med. 2022 Aug 18; Epub ahead of print. [PubMed: 35981225](Description of 2 patients from Washington DC with mpox virus infection and severe pox lesions including proctitis treated with tecovirimat, both responding clinically within 2 days of starting therapy, one patient without any adverse events and one with mild fatigue only; no mention of ALT elevations or hepatotoxicity).
- Desai AN, Thompson GR 3rd, Neumeister SM, Arutyunova AM, Trigg K, Cohen SH. Compassionate use of tecovirimat for the treatment of monkeypox infection. JAMA. 2022;328:1348–1350. [PMC free article: PMC9396467] [PubMed: 35994281](Among 25 adult men with clinically apparent and confirmed mpox virus infection treated with tecovirimat [usually 600 mg twice daily for 14 days], treatment “was generally well tolerated”, adverse events included fatigue, headache, nausea, itching and diarrhea; no mention of ALT elevations or hepatotoxicity).
- See KC. Vaccination for monkeypox virus infection in humans: a review of key considerations. Vaccines (Basel). 2022;10:1342. [PMC free article: PMC9413102] [PubMed: 36016230](Review of the clinical features, epidemiology, prevention and treatment of mpox virus infection discusses tecovirimat, brincidofovir and vaccinia immunoglobulin; mentions that brincidofovir therapy is associated with ALT elevations and has the potential for carcinogenesis, while not mentioning adverse events attributed to tecovirimat or vaccinia immunoglobulin).
- Prevention and treatment of monkeypox. Med Lett Drugs Ther. 2022;64(1658):137–139. [PubMed: 36094551](Concise review on the global outbreak of mpox disease in 2022 that was associated with human-to-human transmission, most frequently in men who have sex with men, and a summary of prevention [vaccination with ACAM2000 or Jynneos, which are live vaccinia virus vaccines approved for prevention of smallpox], and treatment [two agents that are approved as therapy of smallpox which are available under an expanded access protocol for mpox]).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Brincidofovir.[LiverTox: Clinical and Researc...]Review Brincidofovir.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Co-administration of tecovirimat and ACAM2000™ in non-human primates: Effect of tecovirimat treatment on ACAM2000 immunogenicity and efficacy versus lethal monkeypox virus challenge.[Vaccine. 2020]Co-administration of tecovirimat and ACAM2000™ in non-human primates: Effect of tecovirimat treatment on ACAM2000 immunogenicity and efficacy versus lethal monkeypox virus challenge.Russo AT, Berhanu A, Bigger CB, Prigge J, Silvera PM, Grosenbach DW, Hruby D. Vaccine. 2020 Jan 16; 38(3):644-654. Epub 2019 Oct 31.
- Review Therapeutic strategies for human poxvirus infections: Monkeypox (mpox), smallpox, molluscipox, and orf.[Travel Med Infect Dis. 2023]Review Therapeutic strategies for human poxvirus infections: Monkeypox (mpox), smallpox, molluscipox, and orf.De Clercq E, Jiang Y, Li G. Travel Med Infect Dis. 2023 Mar-Apr; 52:102528. Epub 2022 Dec 17.
- Review New Perspectives on Antimicrobial Agents: Tecovirimat for Treatment of Human Monkeypox Virus.[Antimicrob Agents Chemother. 2...]Review New Perspectives on Antimicrobial Agents: Tecovirimat for Treatment of Human Monkeypox Virus.DeLaurentis CE, Kiser J, Zucker J. Antimicrob Agents Chemother. 2022 Dec 20; 66(12):e0122622. Epub 2022 Nov 14.
- Review An overview of tecovirimat for smallpox treatment and expanded anti-orthopoxvirus applications.[Expert Rev Anti Infect Ther. 2...]Review An overview of tecovirimat for smallpox treatment and expanded anti-orthopoxvirus applications.Russo AT, Grosenbach DW, Chinsangaram J, Honeychurch KM, Long PG, Lovejoy C, Maiti B, Meara I, Hruby DE. Expert Rev Anti Infect Ther. 2021 Mar; 19(3):331-344. Epub 2020 Sep 15.
- Tecovirimat - LiverToxTecovirimat - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...