NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The combination of ticarcillin and clavulanate provides an extended-spectrum penicillin with a beta-lactamase inhibitor and was previously used to treat serious bacterial infections due to susceptible organisms. Given parenterally, ticarcillin-clavulanate can cause mild transient aminotransferase elevations, and therapy has been linked to instances of acute cholestatic liver disease similar to that described commonly with amoxicillin-clavulanate (Augmentin).
Background
The combination of ticarcillin, a fourth generation penicillin, and clavulanate combines the extended-spectrum of ticarcillin with beta-lactamase inhibitory activity of clavulanic acid. Neither ticarcillin nor ticarcillin-clavulanate are commercially available in the United States, the combination having been withdrawn from the market in 2015. This combination was indicated for serious infections of the lower respiratory tract, urinary tract, bones and joints and skin. The extended-spectrum of ticarcillin made it an appropriate agent in therapy of Pseudomonas aeruginosa. Ticarcillin also has extended activity against some Enterobacter and Proteus. Ticarcillin is poorly absorbed by mouth and requires parenteral administration. The combination of ticarcillin and clavulanate was approved for use in the United States initially in 1985 under the trade name of Timentin. The combination was provided as 3 grams of ticarcillin with 100 mg of clavulanate which was typically given intravenously every 4 to 6 hours for 5 to 14 days. Common side effects of ticarcillin included nausea, epigastric discomfort, diarrhea, headache, dizziness, rash and hypersensitivity reactions. Rare but potentially serious adverse events included anaphylaxis, hypersensitivity syndrome, renal dysfunction and Stevens Johnson syndrome.
Hepatotoxicity
Intravenous therapy with ticarcillin and clavulanate has been associated with elevations in serum aminotransferase levels in up to 10% of patients; however, these abnormalities were usually subclinical and self-limited. More important were rare instances of acute cholestatic liver injury arising several days to several weeks after initiation of ticarcillin-clavulanate. This hepatic injury resembled that caused by amoxicillin-clavulanate and was probably caused by the beta-lactamase inhibitor rather than the ticarcillin. However, too few cases were described in the literature to define the clinical characteristics of the idiosyncratic liver injury.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the liver injury associated with the combination of ticarcillin and clavulanate was probably hypersensitivity to the clavulanic acid. However, the possibility exists that some instances of hepatotoxicity following this combination were due to ticarcillin.
Outcome and Management
In the few cases of cholestatic liver injury following therapy with ticarcillin-clavulanate that have been described, resolution occurred rapidly in one patient whereas the second died of an underlying disease before recovery was complete. Cases of fatalities and chronic cholestasis have been described after amoxicillin-clavulanate therapy which is a much more commonly prescribed combination.
Drug Class: Antiinfective Agents, Penicillins (Fourth Generation)
Other Drugs in the Class: Piperacillin, Piperacillin-Tazobactam, Ticarcillin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Ticarcillin-Clavulanate – Generic, Timentin®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
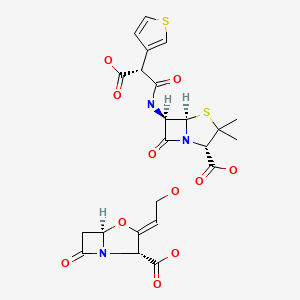
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Ticarcillin- Clavulanic Acid | 86482-18-0 | C15-H16-N2-O6-S2. C8-H9-N-O5 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 20 October 2020
- Zimmerman HJ. Penicillins. In, Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd Ed. Philadelphia: Lippincott, 1999. p. 595-6.(Expert review of penicillins and liver injury published in 1999; piperacillin and ticarcillin are listed as associated with elevations in aminotransferase levels, but without reports of clinically apparent liver injury except with ticarcillin-clavulanate).
- Moseley RH. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 463-82.(Expert review of penicillin induced liver injury mentions that there have been few reports of liver injury due to the extended-spectrum penicillins).
- MacDougall C. Penicillins, cephalosporins, and other β-lactam antibiotics. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1023-38.(Textbook of pharmacology and therapeutics).
- Pines A, Khaja G, Raafat H, Sreedharan KS. Preliminary clinical experience with ticarcillin (BRL 2288) in 101 patients treated for severe respiratory infection. Chemotherapy. 1974;20:39–44. [PubMed: 4210892](Early experience in 101 patients with severe infections given im ticarcillin; local pain was common; minimal and transient elevations in ALT occurred in 4 and Alk P in 5 patients).
- Parry MF, Neu HC. Comparative study of ticarcillin plus tobramycin versus carbenicillin plus gentamicin for the treatment of serious infections due to gram-negative bacilli. Am J Med. 1978;64:961–6. [PubMed: 247895](Comparison of 2 antibiotic combinations in 82 patients with severe gram-negative infections; transient, anicteric ALT elevations [<3 fold increased] occurred in 23% of carbenicillin, but only 3% of ticarcillin treated patients).
- Nelson JD, Kusmiesz H, Shelton S, Woodman E. Clinical pharmacology and efficacy of ticarcillin in infants and children. Pediatrics. 1978;61:858–63. [PubMed: 673548](Among 98 children given ticarcillin iv or im, 3 had AST elevations to 50-81 U/L without jaundice and all values decreased during continued treatment).
- González MA. Comparison of the efficacy and safety of mezlocillin and ticarcillin in the treatment of patients with serious systemic infections. J Antimicrob Chemother. 1982;9 Suppl A:229–30. [PubMed: 6210671](Randomized clinical trial of ticarcillin vs mezlocillin in 34 patients with severe infections; no mention of ALT elevations).
- Graft DF, Chesney PJ. Use of ticarcillin following carbenicillin-associated hepatotoxicity. J Pediatr. 1982;100:497–9. [PubMed: 7062188](Three patients with cystic fibrosis had elevations of ALT levels [to 54, 320 and 445 U/L] during iv carbenicillin therapy, minimally or not at all during subsequent iv ticarcillin therapy).
- Parry MF, Neu HC. The safety and tolerance of mezlocillin. J Antimicrob Chemother. 1982;9 Suppl A:273–80. [PubMed: 6210679](Analysis of 1148 patients given iv mezlocillin for 1-52 days; 3.7% had hypersensitivity reactions, 0.9% elevations in ALT, AST or Alk P, all were reversible and anicteric. In direct comparison, AST elevations occurred in 1.5% of mezlocillin vs 7.4% of ticarcillin recipients).
- Ramírez-Ronda CH, Gutiérrez J, Bermúdez RH. Comparative effectiveness, safety and tolerance of mezlocillin and ticarcillin: a prospective randomized trial. J Antimicrob Chemother. 1982;9 Suppl A:125–9. [PubMed: 6210660](Randomized clinical trial comparing mezlocillin [n=21] and ticarcillin [n=20]; no ALT elevations mentioned).
- Reed WP, Palmer DL. Comparison of azlocillin and ticarcillin in the treatment of urinary tract infection. J Antimicrob Chemother. 1983;11 Suppl B:189–93. [PubMed: 6619028](Randomized clinical trial of azlocillin vs ticarcillin in 35 patients with urinary tract infections, both were highly effective; no mention of ALT elevations or hepatic injury).
- Jansen W, Schwarz A. Comparative evaluation of netilmicin-ticarcillin and tobramycin-ticarcillin in the treatment of serious systemic infections in elderly patients. Clin Ther. 1984;7:112–20. [PubMed: 6394127](Clinical trial in 60 patients with severe infections treated with ticarcillin combined with an aminoglycoside for 4-12 days; 92% efficacy, oto- and nephrotoxicity was attributed to tobramycin, no mention of hepatotoxicity or ALT elevations).
- Van der Auwera P, Legrand JC. Ticarcillin-clavulanic acid therapy in severe infections. Drugs Exp Clin Res. 1985;11:805–13. [PubMed: 3836862](20 patients with severe infections received iv ticarcillin-clavulanate for 3 to 41 days; ALT elevations occurred in 3, but all were mild and self-limited).
- Cone LA, Woodard DR, Stoltzman DS, Byrd RG. Ceftazidime versus tobramycin-ticarcillin in the treatment of pneumonia and bacteremia. Antimicrob Agents Chemother. 1985;28:33–6. [PMC free article: PMC176304] [PubMed: 3899005](Randomized clinical trial of ceftazidime [n=128] vs tobramycin-ticarcillin [n=131] for severe infections; no mention of ALT elevations).
- Mostow SR, O’Brien RF. Safety and effectiveness of ticarcillin plus clavulanate potassium treatment of lower respiratory tract infections. Am J Med. 1985;79:78–80. [PubMed: 4073098](Description of 43 patients treated with ticarcillin-clavulanate; no mention of ALT elevations or liver toxicity).
- Cox CE. Comparative study of ticarcillin plus clavulanate potassium versus piperacillin in the treatment of hospitalized patients with urinary tract infections. Am J Med. 1985;79:88–90. [PubMed: 4073101](Randomized clinical trial in hospitalized patients; 29% of ticarcillin-clavulanate vs 18% piperacillin recipients had laboratory adverse events, mostly ALT elevations, but specific numbers not given).
- Gebhart RJ, Duma RJ, Patterson PM. Timentin in the treatment of symptomatic complicated urinary tract infections in adult patients. Am J Med. 1985;79:101–5. [PubMed: 3852637](Use of iv ticarcillin-clavulanate in 34 patients with urinary tract infection; adverse events occurred in only one patient, no ALT elevations mentioned).
- Pankey GA, Katner HP, Valainis GT, Clarkson MJ, Cortez LM, Dalovisio JR. Overview of bacterial infections of the skin and soft tissue and clinical experience with ticarcillin plus clavulanate potassium in their treatment. Am J Med. 1985;79:106–15. [PubMed: 4073076](Trial in patients with severe skin infections found ALT elevations in 3 of 19 [16%] patients on ticarcillin-clavulanate, but none of 12 on cefazolin).
- Roselle GA, Bode R, Hamilton B, Bibler M, Sullivan R, Douce R, Staneck JL. Clinical trial of the efficacy and safety of ticarcillin and clavulanic acid. Antimicrob Agents Chemother. 1985;27:291–6. [PMC free article: PMC176263] [PubMed: 3888101](43 patients given ticarcillin-clavulanate; 88% cure, 25% adverse events, but no mention of ALT elevations or liver injury).
- Sanders CV, Marier RL, Aldridge KE, Derks FW, Martin DH. Safety and effectiveness of ticarcillin plus clavulanic acid in the treatment of community-acquired acute pyelonephritis in adult women. Am J Med. 1985;79:96–100. [PubMed: 3907345](Use of ticarcillin-clavulanate had poor efficacy in pyelonephritis and 2 of 19 [10%] patients had AST elevations).
- Ryan J, Dudley FJ. Cholestasis with ticarcillin-potassium clavulanate (Timentin). Med J Aust. 1992;156:291. [PubMed: 1738336](75 year old man developed jaundice 31 days after stopping a 9 day course of ticarcillin-clavulanate [bilirubin 8.1 mg/dL, ALT 448 U/L, Alk P 1330 U/L]; died of progressive lymphoma soon thereafter).
- Sweet JM, Jones MP. Intrahepatic cholestasis due to ticarcillin-clavulanate. Am J Gastroenterol. 1995;90:675–6. [PubMed: 7717345](60 year old woman with neutropenic sepsis developed jaundice with 2 days of starting ticarcillin-clavulanate and gentamicin with bilirubin rising to 33 mg/dL, ALT 142 U/L, Alk P 355 U/L, improving on stopping antibiotics, but temporary worsening with restarting ticarcillin-clavulanate, then resolving in 1 month; the role of sepsis in causing the jaundice is suggested by the very short latency after starting ticarcillin-clavulanate).
- Yellin AE, Johnson J, Higareda I, Congeni BL, Arrieta AC, Fernsler D, West J, et al. Ertapenem or ticarcillin/clavulanate for the treatment of intra-abdominal infections or acute pelvic infections in pediatric patients. Am J Surg. 2007;194:367–74. [PubMed: 17693284](Among 105 children in a controlled trial, ALT elevations occurred in 3% on ertapenem vs 4% on ticarcillin-clavulanate; no details given).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, none were attributed to ticarcillin or piperacillin).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](313 cases of drug induced liver injury were seen over a 12 year period at a large hospital in Bangalore, India; none were attributed to extended spectrum, 4th generation penicillins).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, but ticarcillin and piperacillin were not listed in the top 41 causes).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, none of which were attributed to an extended spectrum penicillin).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 15 due to amoxicillin-clavulanate, 1 to dicloxacillin [2nd generation] and 1 to phenoxymethylpenicillin [1st generation], the latter two cases being anicteric; none were attributed to a 4th generation penicillin).
- Sistanizad M, Peterson GM. Drug-induced liver injury in the Australian setting. J Clin Pharm Ther. 2013;38:115–20. [PubMed: 23350857](Among 17 persons with suspected drug induced liver injury seen over at 12 month period at a referral hospital in Tasmania, 11 were attributed to antibiotics including 4 to flucloxacillin, 2 amoxicillin with clavulanate, 2 amoxicillin, and 1 each to rifampin, moxifloxacin and ciprofloxacin; none to 4th generation penicillins).
- Devarbhavi H, Andrade RJ. Drug-induced liver injury due to antimicrobials, central nervous system agents, and nonsteroidal anti-inflammatory drugs. Semin Liver Dis. 2014;34:145–61. [PubMed: 24879980](Review of drug induced liver injury from various classes of agents, mentions that amoxicillin-clavulanate is the leading cause of drug induced liver injury, marked by a latency of several days to weeks, often after stopping the antibiotic, the injury varying from cholestatic to hepatocellular and the mortality rate being as high as 7%; no discussion of the 4th generation penicillins).
- Björnsson ES. Epidemiology and risk factors for idiosyncratic drug-induced liver injury. Semin Liver Dis. 2014;34:115–22. [PubMed: 24879977](Estimates of the incidence of drug induced liver injury have ranged from 2 to 19 case per 100,000 inhabitants, probably because of variation in medication use, definitions used and rigor of capturing all patients in a population; in recent studies, amoxicillin-clavulanate has been the most frequently implicated drug).
- Kaye JA, Castellsague J, Bui CL, Calingaert B, McQuay LJ, Riera-Guardia N, Saltus CW, et al. Risk of acute liver injury associated with the use of moxifloxacin and other oral antimicrobials: a retrospective, population-based cohort study. Pharmacotherapy. 2014;34:336–49. [PMC free article: PMC4260122] [PubMed: 24865821](In a US healthcare database with 1.3 million antimicrobial users, there were 607 cases of liver injury and 11 cases of liver failure, the highest relative risk for current single use being 3.2 for levofloxacin, 2.5 for amoxicillin-clavulanate, 2.5 for doxycycline, 2.3 for moxifloxacin and 2.3 for amoxicillin; no analysis of cases of other penicillins).
- Fontana RJ. Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterology. 2014;146:914–28. [PMC free article: PMC4031195] [PubMed: 24389305](Review of clinical phenotypes and pathogenesis of different forms of drug induced liver injury including antibiotics and amoxicillin-clavulanate and flucloxacillin, but not of other penicillins).
- Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89:95–106. [PubMed: 24388027](Review of drug induced liver injury mentions that antibiotics are the most common cause and amoxicillin-clavulanate the most common single cause in Europe and the US, accounting for 8-22% of cases; no mention of other penicillins).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury from Latin American countries published between 1996 and 2012 identified 176 cases, of which 37 [19%] were attributed to antimicrobials, including one to benzathine penicillin and 3 to amoxicillin-clavulanate, but none to 4th generation penicillins such as piperacillin or ticarcillin).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 323 [36%] were attributed to antibiotics of which 106 [12%] were due to penicillins including one to a 1st, three to a 2nd [all due to oxacillin], 97 to a 3rd [91 to amoxicillin-clavulanate, and 6 to amoxicillin alone], and five to a 4th generation penicillin [all 5 to piperacillin-tazobactam]).
- Guéant JL, Romano A, Cornejo-Garcia JA, Oussalah A, Chery C, Blanca-López N, Guéant-Rodriguez RM, et al. HLA-DRA variants predict penicillin allergy in genome-wide fine-mapping genotyping. J Allergy Clin Immunol. 2015;135:253–9. [PubMed: 25224099](In a genome wide association study of 387 patients with immediate allergic reactions to beta-lactam antibiotics, several class 2 HLA associations [HLA-DRA regions] were found for penicillin responses).
- Björnsson ES. Drug-induced liver injury: an overview over the most critical compounds. Arch Toxicol. 2015;89:327–34. [PubMed: 25618544](Review of the most common causes of drug induced liver injury in 3 recent surveys, all of which listed amoxicillin-clavulanate as the most frequent cause; none listed other penicillins).
- Björnsson ES. Hepatotoxicity by drugs: the most common implicated agents. Int J Mol Sci. 2016;17:224. [PMC free article: PMC4783956] [PubMed: 26861310](Review of the most common causes of drug induced liver injury based upon categorization from LiverTox, amoxicillin-clavulanate but no other penicillin was in the top category of causes [likelihood scores of A, with more than 100 cases published in the literature]).
- Faulkner L, Gibson A, Sullivan A, Tailor A, Usui T, Alfirevic A, Pirmohamed M, et al. Detection of primary T cell responses to drugs and chemicals in HLA-typed volunteers: implications for the prediction of drug immunogenicity. Toxicol Sci. 2016;154:416–29. [PubMed: 27637899](Demonstration of T cell priming to drugs linked with specific class I HLA-alleles).
- Nicoletti P, Aithal GP, Bjornsson ES, Andrade RJ, Sawle A, Arrese M, Barnhart HX, et al. International Drug-Induced Liver Injury Consortium, Drug-Induced Liver Injury Network Investigators, and International Serious Adverse Events Consortium. Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in HLA and other genes in a genome-wide association study. Gastroenterology. 2017;152:1078–89. [PMC free article: PMC5367948] [PubMed: 28043905](A genome wide association study done on 862 Caucasian patients with drug induced liver injury demonstrated a strong link with HLA-A*33:01 in those with cholestatic liver injury, particularly in cases attributed to terbinafine, fenofibrate and ticlopidine).
- Blumenthal KG, Youngster I, Rabideau DJ, Parker RA, Manning KS, Walensky RP, Nelson SB. Peripheral blood eosinophilia and hypersensitivity reactions among patients receiving outpatient parenteral antibiotics. J Allergy Clin Immunol. 2015;136:1288–94.e1. [PMC free article: PMC4640981] [PubMed: 25981739](Among 824 patients who underwent outpatient parenteral antibiotic therapy for at least 2 weeks, 210 [25%] developed eosinophilia including 58 of 207 [28%] who received “penicillins” of whom 3 developed signs of “possible” DRESS syndrome; specific penicillins accounting for the cases were not provided).
- Tailor A, Faulkner L, Naisbitt DJ, Park BK. The chemical, genetic and immunological basis of idiosyncratic drug-induced liver injury. Hum Exp Toxicol. 2015;34:1310–7. [PubMed: 26614821](Review of mechanisms of idiosyncratic drug induced liver injury focusing upon chemically reactive drug metabolites and genetic associations, particularly those with HLA alleles that implicate the adaptive immune response).
- Meng X, Earnshaw CJ, Tailor A, Jenkins RE, Waddington JC, Whitaker P, French NS, et al. Amoxicillin and clavulanate form chemically and immunologically distinct multiple haptenic structures in patients. Chem Res Toxicol. 2016;29:1762–72. [PubMed: 27603302](Analysis of amoxicillin and clavulanate adducts produced in vitro and detected in vivo in patients with amoxicillin-clavulanate hepatotoxicity, the adducts were also present in culture median used to detect reactivity of specific T cell clones from patients).
- Takeuchi Y, Shinozaki T, Kumamaru H, Hiramatsu T, Matsuyama Y. Analyzing intent-to-treat and per-protocol effects on safety outcomes using a medical information database: an application to the risk assessment of antibiotic-induced liver injury. Expert Opin Drug Saf. 2018;17:1071–9. [PubMed: 30252549](Cohort matching of cases with vs without antibiotic therapy in a large electronic medical record database from the University of Tokyo Hospital from 2011 to 2015 with adjustments found rates of liver test abnormalities within 30 days of starting penicillins [25.2 per 1000] was higher than that of fluoroquinolones [11.4] and macrolide antibiotics [8.1] as well as controls [6.5 to 7.1]).
- Cirulli ET, Nicoletti P, Abramson K, Andrade RJ, Bjornsson ES, Chalasani N, Fontana RJ, et al. Drug-Induced Liver Injury Network (DILIN) investigators. International DILI consortium (iDILIC). A missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology. 2019;156:1707–16.e2. [PMC free article: PMC6511989] [PubMed: 30664875](Genome wide association studies on 2048 patients with drug induced liver injury and 12,439 controls identified a variant in PTPN22 which was highly associated with liver injury, allele frequency being 0.12 among cases and 0.08 among controls with highest association in Northern Europeans and in cases of amoxicillin clavulanate, PTPN22 being a cellular kinase involved in modulation of immune reactions).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Beta-lactamase production and susceptibilities to amoxicillin, amoxicillin-clavulanate, ticarcillin, ticarcillin-clavulanate, cefoxitin, imipenem, and metronidazole of 320 non-Bacteroides fragilis Bacteroides isolates and 129 fusobacteria from 28 U.S. centers.[Antimicrob Agents Chemother. 1...]Beta-lactamase production and susceptibilities to amoxicillin, amoxicillin-clavulanate, ticarcillin, ticarcillin-clavulanate, cefoxitin, imipenem, and metronidazole of 320 non-Bacteroides fragilis Bacteroides isolates and 129 fusobacteria from 28 U.S. centers.Appelbaum PC, Spangler SK, Jacobs MR. Antimicrob Agents Chemother. 1990 Aug; 34(8):1546-50.
- In vitro activity of amoxycillin/clavulanate and ticarcillin/clavulanate compared with that of other antibiotics against anaerobic bacteria: comparison with the results of the 1987 survey.[Acta Clin Belg. 1996]In vitro activity of amoxycillin/clavulanate and ticarcillin/clavulanate compared with that of other antibiotics against anaerobic bacteria: comparison with the results of the 1987 survey.Pierard D, De Meyer A, Rosseel P, Van Cauwenbergh M, Struelens MJ, Delmee M, Goossens H, Claeys G, Glupczynski Y, Verbist L, et al. Acta Clin Belg. 1996; 51(2):70-9.
- Beta-lactamase production, beta-lactam sensitivity and resistance to synergy with clavulanate of 737 Bacteroides fragilis group organisms from thirty-three US centres.[J Antimicrob Chemother. 1990]Beta-lactamase production, beta-lactam sensitivity and resistance to synergy with clavulanate of 737 Bacteroides fragilis group organisms from thirty-three US centres.Jacobs MR, Spangler SK, Appelbaum PC. J Antimicrob Chemother. 1990 Sep; 26(3):361-70.
- Review Augmentin (amoxicillin/clavulanate) in the treatment of community-acquired respiratory tract infection: a review of the continuing development of an innovative antimicrobial agent.[J Antimicrob Chemother. 2004]Review Augmentin (amoxicillin/clavulanate) in the treatment of community-acquired respiratory tract infection: a review of the continuing development of an innovative antimicrobial agent.White AR, Kaye C, Poupard J, Pypstra R, Woodnutt G, Wynne B. J Antimicrob Chemother. 2004 Jan; 53 Suppl 1:i3-20.
- Review Penicillins (3rd Generation).[LiverTox: Clinical and Researc...]Review Penicillins (3rd Generation).. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Ticarcillin-Clavulanate - LiverToxTicarcillin-Clavulanate - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...