NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Tramadol is an opioid analgesic used for the therapy of mild-to-moderate pain. Tramadol overdose can cause acute liver failure. Pharmacologic doses of tramadol has not been associated with cases of clinically apparent drug induced liver disease.
Background
Tramadol is a synthetic codeine analog that acts as a weak opioid agonist in addition to mildly inhibiting serotonin and norepinephrine reuptake. Tramadol is effective against mild-to-moderate pain, but is not as effective as standard opioids and not recommended for severe pain. Tramadol was approved for use in the United States in 1995 and is currently widely used, with more than 18 million prescriptions written yearly. Tramadol is available in 50 mg tablets in multiple generic forms and under the brand name Ultram. It is also available as tablets of 37.5 mg in combination with acetaminophen (325 mg) both generically and under the brand name Ultracet. The usual dose in adults is initially 25 mg daily, with titration based on effect and tolerance to 50 to 100 mg every 4 to 6 hours as needed for pain, but not to exceed 400 mg daily. Extended release formulations in capsules of 100, 200 and 300 mg are also available (ConZip and generics) and are given once daily. Physical dependence can occur, and while the potential for abuse is less than with more typical opioid analgesics, tramadol is classified as a schedule IV drug and is provided under a risk evaluation and mitigation strategy (REMS). Common side effects are nausea, dizziness, dry mouth, sedation and headache. Tramadol may increase the risk of seizures. It is considered contraindicated in children below the age of 12 because of risk of respiratory depression, and prolonged use should be avoided in pregnancy because of the risk of infant withdrawal syndrome. Rare but potentially severe adverse events include seizures, severe respiratory depression, serotonin syndrome, addiction, abuse and withdrawal syndrome.
Hepatotoxicity
Serum aminotransferase levels can be elevated in a small proportion of patients receiving tramadol, particularly with high doses. Intentional and accidental overdoses of tramadol can cause respiratory arrest as well as acute liver failure, several fatal instances of which have been reported. In these cases, however, the liver injury may have been caused by shock, hypoxia or ischemia secondary to the respiratory arrest. Liver injury attributed to tramadol overdose has also been associated with hyperammonemia, lactic acidosis and hepatic steatosis, suggestive of direct mitochondrial injury. In some situations, acute liver failure after tramadol overdose may be related to acetaminophen taken separately or in combination with tramadol. Clinically apparent idiosyncratic liver injury with recommended doses of tramadol has not been reported.
Likelihood score: E* (unproven cause of liver injury in therapeutic doses but reported to cause liver injury with overdose).
Mechanism of Injury
The mechanism of hepatotoxicity from overdoses of tramadol is not known, but is likely due to direct hepatocellular injury, either as a result of ischemia or mitochondrial toxicity. Tramadol is metabolized by the liver, predominantly by CYP 2D6 and 3A4 to its active form and it can result in troublesome drug-drug interactions.
Outcome and Management
The minor enzyme elevations that occur with tramadol use are usually mild, asymptomatic and self-limited, resolving even with continuation of therapy. Cases of acute liver failure due to tramadol overdose require intensive medical management and can be fatal. In acute overdoses, there may be interaction with other agents capable of causing liver injury (particularly acetaminophen).
Drug Class: Opioids
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Tramadol – Generic, Ultram®
DRUG CLASS
Opioids
Product labeling at DailyMed, National Library of Medicine, NIH
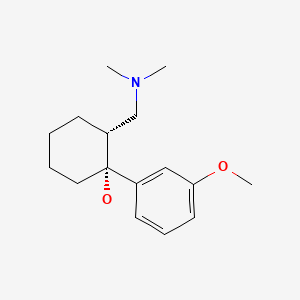
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Tramadol | 27203-92-5 | C16-H25-N-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 24 November 2020
- Zimmerman HJ. Nonsteroidal anti-inflammatory drugs. Drugs used to treat rheumatic and musculospastic disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 517-41.(Textbook of hepatotoxicity published in 1999; tramadol is not discussed).
- Yaksh T, Wallace M. Opioids, analgesia, and pain management. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 355-386.(Textbook of pharmacology and therapeutics).
- Lewis KS, Han NH. Tramadol: a new centrally acting analgesic. Am J Health Syst Pharm. 1997;54:643–52. [PubMed: 9075493](Review of pharmacology, efficacy and side effects of tramadol in management of pain; extensively metabolized in the liver, largely through CYP 2D6; adverse events in 15%, but no mention of hepatic effects).
- Cossmann M, Kohnen C, Langford R, McCartney C. Drugs. 1997;53 Suppl 2:50–62. [Tolerance and safety of tramadol use. Results of international studies and data from drug surveillance] [PubMed: 9190325](Extensive review of side effects of tramadol from phase II-IV studies in 21,000 patients from 1977-93; little evidence of respiratory depression or dependence with oral administration; no mention of hepatotoxicity).
- Bamigbade TA, Langford RM. Tramadol hydrochloride: an overview of current use. Hosp Med. 1998;59:373–6. [PubMed: 9722388](Review of mechanism of action, pharmacology and uses of tramadol).
- Tolman KG. Hepatotoxicity of non-narcotic analgesics. Am J Med. 1998;105:13S–19S. [PubMed: 9715830](Review of liver injury due to analgesics; states that tramadol has not been reported to cause hepatic injury).
- Musshoff F, Madea B. Fatality due to ingestion of tramadol alone. Forensic Sci Int. 2001;116:197–9. [PubMed: 11182272](26 year old man found dead after tramadol overdose; autopsy showed brain edema, no mention of liver).
- Highlights of the 22nd French pharmacovigilance meeting. Prescrire Int. 2002;11:21–3. [PubMed: 11985373](Tramadol reported to interact with warfarin leading to marked increases in INR and bleeding).
- Loughrey MB, Loughrey CM, Johnston S, O'Rourke D. Fatal hepatic failure following accidental tramadol overdose. Forensic Sci Int. 2003;134:232–3. [PubMed: 12850423](Accidental tramadol overdose in 67 year old man taking ~1000 mg daily for 8 days, presenting with respiratory failure, stupor and liver injury [bilirubin 3.3 mg/dL, ALT 1739 U/L, Alk P 89 U/L, lactic acidosis and hypoglycemia], dying after cardiopulmonary arrest and possibly ischemic hepatic injury).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to tramadol).
- De Decker K, Cordonnier J, Jacobs W, Coucke V, Schepens P, Jorens PG. Fatal intoxication due to tramadol alone: case report and review of the literature. Forensic Sci Int. 2008;175:79–82. [PubMed: 17875377](28 year old man with acute tramadol overdose suffered cardiopulmonary arrest, was resuscitated, but died two days later with acute liver and renal failure and hyperammonemia; liver biopsy showed steatosis and centrolobular ischemic necrosis).
- Leppert W. Tramadol as an analgesic for mild to moderate cancer pain. Pharmacol Rep. 2009;61:978–92. [PubMed: 20081232](Review of mechanism of action, pharmacology, efficacy and safety of tramadol in mild-to-moderate cancer pain; side effects occur in 1-6% of patients, but serious adverse effects are very rare; hepatotoxicity and ALT abnormalities are not discussed).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, none of which were attributed to tramadol).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to tramadol).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to tramadol, other opiates or opiate antagonists).
- FDA warns against use of codeine and tramadol in children and breastfeeding women. Med Lett Drugs Ther. 2017;59(1521):86–8. [PubMed: 28520700](Concise summary of revised FDA recommendations on use of tramadol in children listing it as contraindicated in those below the age of 12 because of risk of severe respiratory depression, cautioning its use in adolescents because of addiction and abuse, and avoidance of prolonged use in pregnant women because of potential of withdrawal syndrome in newborns; no mention of ALT elevations or hepatotoxicity).
- Opioids for pain. Med Lett Drugs Ther. 2018;60(1544):57–64. [PubMed: 29664446](Concise review of the mechanism of action, efficacy, safety and costs of opioids used for pain relief, discusses adverse events or short and long term use of tramadol, but makes no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Opioids.[LiverTox: Clinical and Researc...]Review Opioids.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review [Tramadol in acute pain].[Drugs. 1997]Review [Tramadol in acute pain].Lehmann KA. Drugs. 1997; 53 Suppl 2:25-33.
- Review "Weak" opioid analgesics. Codeine, dihydrocodeine and tramadol: no less risky than morphine.[Prescrire Int. 2016]Review "Weak" opioid analgesics. Codeine, dihydrocodeine and tramadol: no less risky than morphine.. Prescrire Int. 2016 Feb; 25(168):45-50.
- Review Tramadol with or without paracetamol (acetaminophen) for cancer pain.[Cochrane Database Syst Rev. 2017]Review Tramadol with or without paracetamol (acetaminophen) for cancer pain.Wiffen PJ, Derry S, Moore RA. Cochrane Database Syst Rev. 2017 May 16; 5(5):CD012508. Epub 2017 May 16.
- The problem of pain: Additive analgesic effect of tramadol and buprenorphine in a patient with opioid use disorder.[Subst Abus. 2019]The problem of pain: Additive analgesic effect of tramadol and buprenorphine in a patient with opioid use disorder.Montalvo C, Genovese N, Renner J. Subst Abus. 2019; 40(2):136-139. Epub 2019 Mar 5.
- Tramadol - LiverToxTramadol - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...