NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Trifluridine/tipiracil is the combination of an antineoplastic pyrimidine analogue (trifluridine) with an inhibitor of its metabolism (tipiracil) that is used in the therapy of refractory, metastatic colorectal cancer. Trifluridine/tipiracil is associated with a low rate of transient serum enzyme elevations during therapy, but has not been implicated in cases of clinically apparent acute liver injury with jaundice.
Background
Trifluridine (trye flure' i deen)/tipiracil (tye pir’ a sil) combines an antineoplastic pyrimidine analogue (2-deoxy-5-trifluoromethyl uridine) with a thymidine phosphorylase inhibitor that blocks its rapid metabolism, thus increasing the bioavailability of trifluridine. Trifluridine is absorbed orally and converted intracellularly to a triphosphate which becomes incorporated into DNA causing inhibition of DNA synthesis, a decrease in cellular growth and proliferation and triggering apoptosis. Trifluridine triphosphate is metabolized by thymidine phosphorylase which is inhibited by tipiracil, thus providing a longer half-life and increased intracellular concentrations of active phosphorylated trifluridine. Trifluridine/tipiracil was shown to have potent activity in vitro in cellular and in vivo in animal models of colon cancer. In clinical trials, this combination led to prolongation of progression-free as well as overall survival in patients with previously treated, refractory metastatic colorectal cancer. Trifluridine/tipiracil was approved for use in the United States in 2015 and is available in tablets of 15 mg/14 mg and 20 mg/19 mg under the commercial name Lonsurf. Current indications are for refractory, metastatic colorectal cancer after previous treatment with other agents. The recommended dose regimen is 35 mg/m2 (based upon the trifluridine component) orally twice daily on days 1 through 5 and days 8 through 12 of each 28-day cycle. Common side effects include bone marrow suppression, weakness, fatigue, nausea, anorexia, diarrhea, abdominal pain and fever. Less common, but potentially severe adverse events include severe myelosuppression, febrile neutropenia, infections, sepsis and embryo-fetal toxicity.
Hepatotoxicity
Pooled analyses of preregistration clinical trials reported that serum enzyme elevations occurred in up to 24% of patients on trifluridine/tipiracil therapy, but were also elevated in 27% of controls. Similarly, ALT values above 5 times ULN occurred in 2% of trifluridine/tipiracil treated compared to 4% of placebo treated subjects. In these and subsequent studies, clinically apparent liver adverse reactions attributed to trifluridine/tipiracil were not reported.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Hepatotoxicity from trifluridine/tipiracil appears to be rare and liver test abnormalities are most likely to be due to the underlying condition rather than the drug itself. Trifluridine is metabolized in most cells by thymidine phosphorylase to 5-trilfuorosmethyluracil, which is inactive. Tipiracil is excreted largely unchanged in the urine. Neither agent appears to affect hepatic cytochrome P450 activities.
Outcome and Management
The serum aminotransferase and alkaline phosphatase elevations that occur during trifluridine/tipiracil therapy are usually transient, asymptomatic and mild. Trifluridine/tipiracil has not been linked to cases of acute liver failure, chronic hepatitis or vanishing bile duct syndrome. There is no information on cross sensitivity to hepatic injury between trifluridine/tipiracil and other pyrimidine analogues used in cancer chemotherapy such as capecitabine, gemcitabine and fluorouracil.
Drug Class: Antineoplastic Agents
Other Drugs in the Subclass, Pyrimidine Analogues: Azacitidine, Capecitabine, Cytarabine, Decitabine, Floxuridine, Fluorouracil, Gemcitabine
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Trifluridine/Tipiracil – Lonsurf®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Trifluridine | 70-00-8 | C10-H11-F3-N2-O5 |
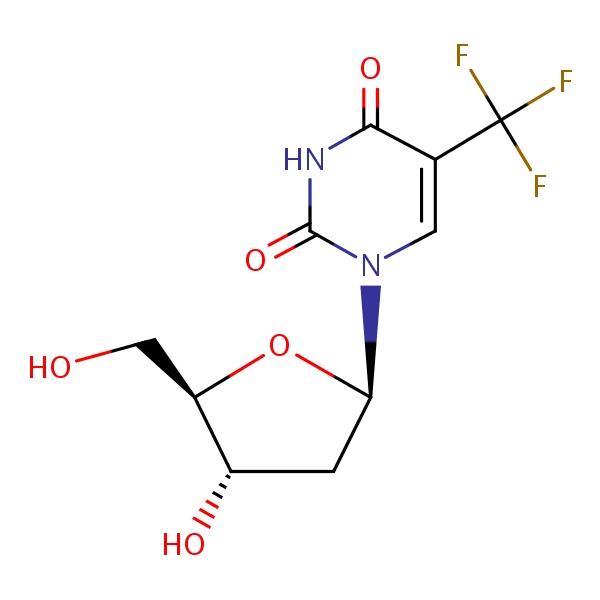 |
| Tipiracil | 183204-74-2 | C9-H11-Cl-N4-O2 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 16 April 2017
- Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999 before the availability of trifluridine/tipiracil).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3nd ed. Amsterdam: Elsevier, 2013, p. 541-67.(Review of hepatotoxicity of cancer chemotherapeutic agents; trifluridine/tipiracil is not discussed).
- Chabner BA, Barnes J, Neal J, Olson E, Mujagic H, Sequist L, Wilson W, Longo DL, Mitsiades C, Richardson P. Cytotoxic agents. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1677-1730.(Textbook of pharmacology and therapeutics).
- Yoshino T, Mizunuma N, Yamazaki K, Nishina T, Komatsu Y, Baba H, Tsuji A, et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol 2012; 13: 993-1001. [PubMed: 22951287](Among 169 Japanese patients with refractory, metastatic colorectal cancer treated with trifluridine/tipiracil or placebo, overall survival was improved [9.0 vs 6.6 months], but serious adverse events were more frequent with trifluridine [19% vs 9%]; no mention of ALT elevations or hepatotoxicity).
- Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, et al.; RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015; 372: 1909-19. [PubMed: 25970050](Among 800 patients with refractory, metastatic colorectal cancer treated with trifluridine/tipiracil or placebo, overall survival was improved [7.3 vs 5.5 months] and adverse events more frequent with trifluridine included nausea, vomiting, anorexia, fatigue, diarrhea and fever, while ALT elevations occurred in 24% on trifluridine vs 22% on placebo and were above 5 times ULN in 2% vs 4%).
- In brief: trifluridine/tipiracil (Lonsurf) for metastatic colorectal cancer. Med Lett Drugs Ther 2016; 58 (1496): e77. [PubMed: 27249100](Concise review of the mechanism of action, clinical efficacy, safety and costs of trifluridine/tipiracil shortly after its approval for use in the US; mentions common side effects, but not ALT elevations or hepatotoxicity).
- Kotani D, Shitara K, Kawazoe A, Fukuoka S, Kuboki Y, Bando H, Okamoto W, et al. Safety and Efficacy of trifluridine/tipiracil monotherapy in clinical practice for patients with metastatic colorectal cancer: experience at a single institution. Clin Colorectal Cancer 2016; 15: e109-15. [PubMed: 26723516](Among 55 patients with refractory, metastatic colorectal cancer treated with trifluridine/tipiracil, common severe adverse events included neutropenia and infection; no mention of ALT elevations or hepatotoxicity).
- Sueda T, Sakai D, Kudo T, Sugiura T, Takahashi H, Haraguchi N, Nishimura J, et al. Efficacy and safety of regorafenib or TAS-102 in patients with metastatic colorectal cancer refractory to standard therapies. Anticancer Res 2016; 36: 4299-306. [PubMed: 27466548](In a retrospective analysis of patients with metastatic colorectal cancer treated with regorafenib [n=23] or trifluridine/tipiracil [n=14], survival rates were similar [5.8 vs 6.3 months], but adverse events were more frequent with regorafenib including hepatotoxicity [52% vs 7%]).
- Burness CB, Duggan ST. Trifluridine/tipiracil: a review in metastatic colorectal cancer. Drugs 2016; 76 (14): 1393-402. [PubMed: 27568360](Review of the pharmacology, clinical efficacy and safety of trifluridine/tipiracil as therapy of metastatic colorectal cancer; mentions that the rates of total as well as serious adverse events were similar in trifluridine- as in placebo-treated subjects; no mention of ALT elevations or hepatotoxicity).
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Adherence, Dosing, and Managing Toxicities With Trifluridine/Tipiracil (TAS-102).[Clin Colorectal Cancer. 2017]Review Adherence, Dosing, and Managing Toxicities With Trifluridine/Tipiracil (TAS-102).Lee JJ, Chu E. Clin Colorectal Cancer. 2017 Jun; 16(2):85-92. Epub 2017 Jan 25.
- Early Clinical Experience with Trifluridine/Tipiracil for Refractory Metastatic Colorectal Cancer: The ROS Study.[Cancers (Basel). 2021]Early Clinical Experience with Trifluridine/Tipiracil for Refractory Metastatic Colorectal Cancer: The ROS Study.García-Alfonso P, Muñoz A, Jiménez-Castro J, Jiménez-Fonseca P, Pericay C, Longo-Muñoz F, Reyna-Fortes C, Argilés-Martínez G, González-Astorga B, Gómez-Reina MJ, et al. Cancers (Basel). 2021 Sep 8; 13(18). Epub 2021 Sep 8.
- Review Trifluridine/Tipiracil: A Review in Metastatic Colorectal Cancer.[Drugs. 2016]Review Trifluridine/Tipiracil: A Review in Metastatic Colorectal Cancer.Burness CB, Duggan ST. Drugs. 2016 Sep; 76(14):1393-402.
- Trifluridine/tipiracil in combination with local therapy may be a favorable option for refractory metastatic colorectal cancer patients: A case report.[Medicine (Baltimore). 2020]Trifluridine/tipiracil in combination with local therapy may be a favorable option for refractory metastatic colorectal cancer patients: A case report.Lin YL, Liu KL, Lin BR. Medicine (Baltimore). 2020 Oct 23; 99(43):e22780.
- Propensity Score Analysis of Regorafenib Versus Trifluridine/Tipiracil in Patients with Metastatic Colorectal Cancer Refractory to Standard Chemotherapy (REGOTAS): A Japanese Society for Cancer of the Colon and Rectum Multicenter Observational Study.[Oncologist. 2018]Propensity Score Analysis of Regorafenib Versus Trifluridine/Tipiracil in Patients with Metastatic Colorectal Cancer Refractory to Standard Chemotherapy (REGOTAS): A Japanese Society for Cancer of the Colon and Rectum Multicenter Observational Study.Moriwaki T, Fukuoka S, Taniguchi H, Takashima A, Kumekawa Y, Kajiwara T, Yamazaki K, Esaki T, Makiyama C, Denda T, et al. Oncologist. 2018 Jan; 23(1):7-15. Epub 2017 Sep 11.
- Trifluridine - LiverToxTrifluridine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...