NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Viekira Pak is a combination of oral antiviral agents that previously was used to treat chronic hepatitis C, genotype 1. This combination was associated with a low rate of serum enzyme elevations during therapy, and was reported to cause rare cases of clinically apparent liver injury with jaundice and which could result in hepatic decompensation largely in patients with preexisting cirrhosis.
Background
The hepatitis C virus is a small RNA virus that is a major cause of chronic hepatitis, cirrhosis and hepatocellular carcinoma in the United States as well as worldwide. Various approaches to antiviral therapy of chronic hepatitis C have been developed, starting in the 1980s with interferon alfa which was replaced in the 1990s by long acting forms of interferon (peginterferon), to which was added the oral nucleoside analogue, ribavirin. Between 2010 and 2015, several potent oral, direct acting anti-HCV agents were developed and combinations of these found to have marked activity against the virus, allowing for highly effective and well tolerated therapy without use of interferon and with treatment courses of 8, 12 or 24 weeks only.
Viekira Pak (vee kee' rah pak) is the commercial name for a combination of oral, direct acting antiviral agents used to treat chronic hepatitis C associated with HCV genotype 1. The hepatitis C virus (HCV) encodes several nonstructural (NS) polypeptides that are essential for its replication: NS3/4 that has protease and helicase activities, NS5A that is a membrane bound polypeptide that is essential in the creation of the replicative complex, and NS5B an HCV specific, RNA-dependent, RNA polymerase. These polypeptides are effective targets for antiviral therapy of hepatitis C. Viekira Pak was a combination paritaprevir (par’ i ta’ pre veer: formerly ABT-450) which is a potent HCV NS3/4 protease inhibitor, ombitasvir (om bit’ as veer: ABT-267) an NS5A replication complex inhibitor, and dasabuvir (da sa’ bue veer: ABT-333) a nonnucleoside HCV RNA polymerase [NS5B] inhibitor. Paritaprevir is metabolized by CYP 3A4 and is typically given in combination with low doses of ritonavir, an inhibitor of CYP 3A4, to achieve higher and more prolonged drug levels which allow for once daily dosing. In cell culture and in humans infected with HCV, each of the agents had potent activity against HCV, but development of antiviral resistance rapidly arose with continued exposure. The combination of several direct acting agents with different molecular targets allows for a sustained viral suppression while avoiding antiviral resistance. The combination of these three agents (and ritonavir) with and without ribavirin (an antiviral nucleoside analogue with activity against HCV) has been shown to be very effective in suppressing HCV replication in patients infected with HCV genotype 1, and to result in a sustained virological response (SVR) and eradication of HCV in more than 90% of patients when given for 12 weeks or more. Viekira Pak was approved for use in the United States in 2015, the second all-oral antiviral combination to receive approval for chronic hepatitis C. It was available as two tablets, one being the fixed combination of ombitasvir (12.5 mg), paritaprevir (75 mg) and ritonavir (100 mg) which was given once daily, and the other being dasabuvir (250 mg) which was given twice daily with meals. Ribavirin (if a part of the combination therapy as was recommended for genotype 1a and for patients with cirrhosis) was available in tablets of 200 mg and is given twice daily for a total dose of 1,000 mg (if body weight is <75 kg) or 1,200 mg (if body weight ≥75 kg). The indications for Viekira Pak (the combination of dasabuvir, ombitasvir and paritaprevir with ritonavir: D-O-P/r) were limited to patients with HCV genotype 1. The combination of just ombitasvir and paritaprevir with ritonavir (O-P/r) was also available under the commercial name Technive and was approved for use in combination with ribavirin in patients with chronic hepatitis C, genotype 4, without cirrhosis. Side effects of Viekira Pak and Technive were uncommon, and generally mild including nausea, itching, rash, cough and insomnia. When given with ribavirin, side effects were greater, but are largely due to the hemolysis, nasal congestion and skin reactions that are common with that agent. Viekira Pak and Technive were withdrawn by the sponsor from availability in 2018 when combination therapies with greater efficacy and tolerability became available (such as Mavyret, Epclusa and Zepatier).
Hepatotoxicity
In large randomized controlled trials, serum aminotransferase elevations more than 5 times the upper limit of normal (ULN) occurred in 1% to 2% of Viekira Pak treated patients. Interestingly, this rate was lower than occurred with placebo therapy (3% to 7%). The elevations were generally asymptomatic and short lived, resolving with or without dose modification and requiring drug discontinuation in approximately 1% of patients. Despite the occurrence of serum enzyme elevations during therapy, clinically apparent liver injury was rarely reported in preregistration studies. However, during the years of its clinical use in the United States and elsewhere, occasional instances of marked serum aminotransferase elevations with symptoms and mild jaundice were reported, although rarely described in the published literature. Furthermore, some patients with chronic hepatitis C and advanced cirrhosis developed sudden hepatic decompensation during therapy with D-O-P/r. Similar episodes have been described in patients receiving other oral antiviral combinations such as sofosbuvir with daclatasvir, ledipasvir or simeprevir. Thus, this phenomenon may be unrelated to a specific agent, but rather common to all potent antiviral therapies for hepatitis C and perhaps is a paradoxical response to sudden clearance of HCV. Alternatively, these episodes may be spontaneous, coincidental and unrelated to the antiviral therapy. Trials of these therapies in patients with cirrhosis were not placebo controlled so that the rate of spontaneous hepatic decompensation in patients with cirrhosis due to hepatitis C is not well defined. Whatever the reason, the occurrence of decompensation in up to 10% of patients with cirrhosis undergoing potent antiviral therapy makes prospective monitoring advisable and prompt discontinuation of treatment if evidence of hepatic failure supervenes.
Thus, the five antiviral compounds included in Viekira Pak regimens (dasabuvir, ombitasvir, paritaprevir, ritonavir and ribavirin) have been linked to instances of sudden ALT elevations during therapy, but uncommonly to clinically apparent liver injury. In patients with preexisting cirrhosis, antiviral therapy with Viekira Pak has been linked to episodes of lactic acidosis and hepatic decompensation. The cause of these sudden, severe adverse events is unknown but they are usually severe and life threatening, requiring prompt discontinuation of treatment, intensive care management and consideration of emergency liver transplantation. These serious adverse effects appear to be less frequent with more recently developed antiviral regimens for hepatitis C and were another reason for the withdrawal of Viekira Pak and Technive.
Likelihood score: B (likely cause of liver injury arising in patients with pre-existing cirrhosis).
Mechanism of Injury
The mechanism by which Viekira Pak might cause liver injury is not known. The multiple antiviral agents in this combination regimen are metabolized in the liver largely via the cytochrome P450 system, and liver injury may be due to production of a toxic or immunogenic metabolite. Viekira Pak is also susceptible to multiple drug-drug interactions with strong inducers or inhibitors of CYP 3A4, and careful attention to concomitant medications was recommended during use of this regimen.
Outcome and Management
While therapy with Viekira Pak can be associated with mild-to-moderate serum aminotransferase elevations, it has been only rarely linked to cases of clinically apparent liver injury. Nevertheless, monitoring of serum aminotransferase levels monthly during the first 6 months and every 3 months thereafter was recommended. Patients with preexisting cirrhosis were advised to have close monitoring particularly during the first month of treatment. Viekira Pak should be permanently discontinued if jaundice or symptoms of liver injury arise or if serum ALT or AST levels rise and remain above 5 times the ULN.
Drug Class: Antiviral Agents, Hepatitis C Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Dasabuvir, Ombitasvir, Paritaprevir and Ritonavir – Viekira Pak®
Ombitasvir, Paritaprevir and Ritonavir – Technive®
DRUG CLASS
Hepatitis C Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
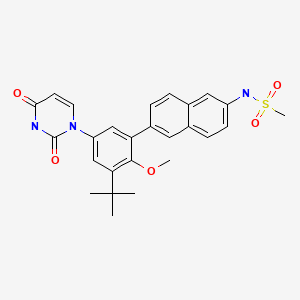
| Dasabuvir | 1132935-63-7 | C26-H27-N3-O5-S |
|
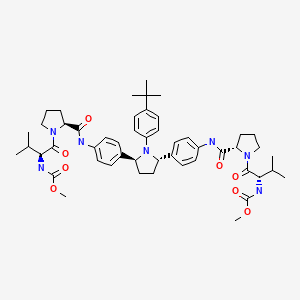
| Ombitasvir | 1258226-87-7 | C50-H67-N7-O8 |
|
| Paritaprevir | 1216941-48-8 | C40-H43-N7-O7-S |

|
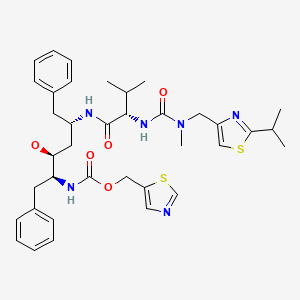
| Ritonavir | 155213-67-5 | C37-H48-N6-O5C37-H48-N6-O5-S2-S2 |
|
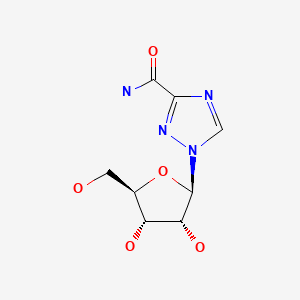
| Ribavirin | 36791-04-5 | C8-H12-N4-O5 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 07 February 2022
Abbreviation used: SVR, sustained virological response.
- Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013.(Multi-authored textbook of hepatotoxicity published in 2013 does not discuss oral, direct acting antiviral agents used to treat hepatitis C).
- Poordad F, Lawitz E, Kowdley KV, Cohen DE, Podsadecki T, Siggelkow S, Heckaman M, et al. Exploratory study of oral combination antiviral therapy for hepatitis C. N Engl J Med. 2013;368:45–53. [PubMed: 23281975](Among 50 patients with chronic hepatitis C, genotype 1, treated with dasabuvir, paritaprevir/ritonavir and ribavirin in varying doses for 12 weeks, the sustained virologic response [SVR] rate was 72%; 1 patient rapidly developed ALT levels above 5 times ULN [308 U/L] without jaundice or symptoms and therapy was stopped at 2 weeks).
- DeGoey DA, Randolph JT, Liu D, Pratt J, Hutchins C, Donner P, Krueger AC, et al. Discovery of ABT-267, a pan-genotypic inhibitor of HCV NS5A. J Med Chem. 2014;57:2047–57. [PubMed: 24400777](Description of identification of an N-phenylpyrrolidine based inhibitor of NS5A, compound 38 [ombitasvir] in screening of HCV replicon systems verified for activity against HCV by phase 1, 3 day studies in 3 patients with chronic hepatitis C, genotype 1).
- Kowdley KV, Lawitz E, Poordad F, Cohen DE, Nelson DR, Zeuzem S, Everson GT, et al. Phase 2b trial of interferon-free therapy for hepatitis C virus genotype 1. N Engl J Med. 2014;370:222–32. [PubMed: 24428468](Among 571 noncirrhotic patients with chronic hepatitis C, genotype 1, treated with 14 different regimens of D-O-P/r with ribavirin for 8, 12 or 24 weeks, SVR rates ranged from 83% to 100%; side effects included ALT elevations above 5 times ULN [peak 408 U/L] in 5 patients [1%], but all resolved without dose modifications and no patient developed clinically apparent liver injury).
- Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–603. [PubMed: 24720703](Among 631 patients with noncirrhotic chronic hepatitis C, genotype 1, treated with Viekira Pak [D-O-P/r]) with ribavirin vs all placebos for 12 weeks, SVR rates were 96% vs 0%, and side effects that were more frequent with antiviral therapy were nausea, pruritus, insomnia, diarrhea and weakness, while rates of ALT elevations above 5 times ULN were less common(0.9% vs 4.4%), and no patient developed clinically apparent acute liver injury).
- Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, Bourlière M, Sulkowski MS, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1604–14. [PubMed: 24720679](Among 394 previously treated, noncirrhotic patients with chronic hepatitis C, genotype 1, treated with Viekira Pak [D-O-P/r] with ribavirin vs placebos for 12 weeks, the SVR rates were 96% vs 0%; adverse events more frequent with D-O-P/r were fatigue, weakness, insomnia, pruritus, cough and anemia; ALT elevations above 5 times ULN occurred in 5 patients [1.7%] on therapy, one of whom stopped therapy early vs 3 [3.1%] on placebo; no patient developed clinically apparent liver injury).
- Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, Tam E, et al. PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–92. [PubMed: 24795200](Among 724 noncirrhotic patients with chronic hepatitis C treated with Viekira Pak [D-O-P/r] with vs without ribavirin for 12 weeks, SVR rates were 99% vs 99% for genotype 1b and 97% vs 90% for genotype 1a; common adverse events were fatigue, headache and nausea while ALT elevations above 5 times ULN occurred in only 4 patients [0.5%]).
- Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, Shiffman ML, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973–82. [PubMed: 24725237](Among 380 cirrhotic patients with chronic hepatitis C, genotype 1, treated with Viekira Pak [D-O-P/r] with ribavirin for 12 vs 24 weeks, SVR rates were 92% vs 96%; ALT elevations above 5 times ULN occurred in 6 patients [1.6%], one of whom had “acute hepatitis” and discontinued treatment early).
- Andreone P, Colombo MG, Enejosa JV, Koksal I, Ferenci P, Maieron A, Müllhaupt B, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147:359–365.e1. [PubMed: 24818763](Among 179 noncirrhotic, previously treated patients with chronic hepatitis C, genotype 1b, treated with Viekira Pak [D-O-P/r] with vs without ribavirin for 12 weeks, SVR rates were 97% vs 100%; significant bilirubin elevations occurred only in those on ribavirin and no patient developed ALT elevations above 5 times ULN).
- Kwo PY, Mantry PS, Coakley E, Te HS, Vargas HE, Brown R Jr, Gordon F, et al. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med. 2014;371:2375–82. [PubMed: 25386767](Among 34 patients with recurrent HCV after liver transplantation who were treated with Viekira Pak [D-O-P/r] and ribavirin for 24 weeks, 97% had an SVR and common adverse events were fatigue, headache, cough and need for cyclosporine dose modification; no patient developed ALT elevations above 5 times ULN).
- Liang TJ, Ghany MG. Therapy of hepatitis C--back to the future. N Engl J Med. 2014;370:2043–7. [PMC free article: PMC6324541] [PubMed: 24795199](Commentary on the evolving status of therapy of chronic hepatitis C from poorly effective and tolerated interferon based treatments to very effective and well tolerated all-oral regimens that yield response rates of 85% to 100% with 12 to 24 weeks of treatment).
- Lawitz E, Sullivan G, Rodriguez-Torres M, Bennett M, Poordad F, Kapoor M, Badri P, et al. Exploratory trial of ombitasvir and ABT-450/r with or without ribavirin for HCV genotype 1, 2, and 3 infection. J Infect. 2015;70:197–205. [PubMed: 25246359](Among 61 previously untreated, noncirrhotic patients with chronic hepatitis C treated with ombitasvir and paritaprevir with ritonavir [O-P/r] with or without ribavirin, SVR rates varied by genotype; 2 patients had ALT elevations above 5 times ULN, but were without symptoms or jaundice, and abnormalities resolved upon stopping).
- Hézode C, Asselah T, Reddy KR, Hassanein T, Berenguer M, Fleischer-Stepniewska K, Marcellin P, et al. Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment-naive and treatment-experienced patients with genotype 4 chronic hepatitis C virus infection(PEARL-I): a randomised, open-label trial. Lancet. 2015;385(9986):2502–9. [PubMed: 25837829](Among 135 patients with chronic hepatitis C, genotype 4, treated with Technive [O-P/r] with vs without ribavirin for 12 weeks, SVR rates were 100% vs 90%, and no patient developed ALT elevations above 5 times ULN or clinically apparent liver injury).
- Sulkowski MS, Eron JJ, Wyles D, Trinh R, Lalezari J, Wang C, Slim J, et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA. 2015;313:1223–31. [PubMed: 25706092](Among 63 patients with chronic hepatitis C, genotype 1, and HIV coinfection who were treated with Viekira Pak [D-O-P/r], SVR rates were 94% [12 weeks] and 91% [24 weeks] and no patient had an ALT elevation above 5 times ULN).
- Kalafateli M, Dusheiko G, Manousou P. Clinical decompensation after achieving SVR with sofosbuvir, daclatasvir and ribavirin in a patient with recurrent HCV post-liver transplant. J Gastrointestin Liver Dis. 2015;24:257–8. [PubMed: 26114189](33 year old male with hemophilia and chronic hepatitis C, genotype 3, underwent liver transplantation and had recurrence of HCV posttransplant, subsequently failing to respond to several interferon based courses of therapy, eventually responding to sofosbuvir, daclatasvir and ribavirin, but then developing hepatic decompensation 2 months after achieving an SVR).
- A 4-drug combination (Viekira Pak) for hepatitis C. Med Lett Drugs Ther. 2015;57(1461):15–7. [PubMed: 25629810](Concise summary of clinical efficacy, side effects, drug-drug interactions and costs of Viekira Pak [D-O-P/r] for chronic hepatitis C, genotype 1, shortly after its approval in the US mentions that ALT elevations occur in 1-4% of patients and may require early discontinuation, for which reason ALT monitoring is recommended for the first 4 weeks).
- Lalezari J, Sullivan JG, Varunok P, Galen E, Kowdley KV, Rustgi V, Aguilar H, et al. Ombitasvir/ paritaprevir/r and dasabuvir plus ribavirin in HCV genotype 1-infected patients on methadone or buprenorphine. J Hepatol. 2015;63:364–9. [PubMed: 25839406](Among 38 noncirrhotic patients with chronic hepatitis C, genotype 1, on opioid replacement therapy who were treated with Viekira Pak [D-O-P/r] with ribavirin for 12 weeks, the SVR rate was 97% and no patient had a serious liver related adverse event or stopped therapy because of ALT elevations; ALT results not provided).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–1352.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 12 were attributed to antiviral agents, but all were antiretroviral agents and none were oral direct acting agents used to treat chronic hepatitis C).
- Bailly F, Pradat P, Virlogeux V, Zoulim F. Antiviral therapy in patients with hepatitis C virus-induced cirrhosis. Dig Dis. 2015;33:613–23. [PubMed: 26159282](Review of the status of antiviral therapy of chronic hepatitis C with cirrhosis summarizing the high rate of adverse events, including hepatic decompensation and death with peginterferon based regimens combined with boceprevir or telaprevir, and recommending the use of the more effective and better tolerated all-oral regimens).
- Ferenci P, Kozbial K, Mandorfer M, Hofer H. HCV targeting of patients with cirrhosis. J Hepatol. 2015;63:1015–22. [PubMed: 26100497](Review of the status of antiviral therapy of chronic hepatitis C with cirrhosis, suggests that genotype 1 infected patients should receive an all-oral regimen such as sofosbuvir with ledipasvir or daclatasvir or the triple combination of dasabuvir with ombitasvir and paritaprevir/ritonavir [D-O-P/r], the major issues being duration of therapy and the role of ribavirin).
- Feld JJ, Moreno C, Trinh R, Tam E, Bourgeois S, Horsmans Y, Elkhashab M, et al. Sustained virologic response of 100% in HCV genotype 1b patients with cirrhosis receiving ombitasvir/paritaprevir/r and dasabuvir for 12 weeks. J Hepatol. 2016;64:301–7. [PubMed: 26476290](Among 60 patients with chronic hepatitis C, genotype 1b, and cirrhosis treated with D-O-P/r for 12 weeks, all achieved an SVR and “laboratory abnormalities were infrequently observed and not clinically significant”; only one patient had a severe adverse event (hypotension and syncope) and 1 had an ALT elevation above 5 times ULN, but on one occasion only, resolving without dose modification).
- Klibanov OM, Gale SE, Santevecchi B. Ombitasvir/paritaprevir/ritonavir and dasabuvir tablets for hepatitis C virus genotype 1 infection. Ann Pharmacother. 2015;49:566–81. [PubMed: 25680759](Review of the pharmacology, efficacy and safety of D-O-P/r with [genotype 1a] or without [genotype 1b] ribavirin in patients with chronic hepatitis C summarizes adverse event profile in a pooled analysis of 2887 patients, and mentions that ALT elevations are rare [≤1.2%] and that isolated bilirubin elevations occur [≤4.5%], but without concurrent ALT elevations; no mention of hepatic decompensation).
- European Association for Study of Liver. EASL recommendations on treatment of hepatitis C 2015. J Hepatol. 2015;63:199–236. [PubMed: 25911336](Guidelines for the antiviral therapy of chronic hepatitis C from the European liver disease research and academic society).
- AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932–54. [PubMed: 26111063](Guidelines for the antiviral therapy of chronic hepatitis C from the US liver and infectious diseases research and academic societies).
- Dyson JK, Hutchinson J, Harrison L, Rotimi O, Tiniakos D, Foster GR, Aldersley MA, et al. Liver toxicity associated with sofosbuvir, an NS5A inhibitor and ribavirin use. J Hepatol. 2016;64(1):234–8. [PubMed: 26325535](74 year old man and 36 year old woman with HCV related cirrhosis developed worsening hepatic decompensation within a few weeks of starting sofosbuvir, an NS5A inhibitor and ribavirin [peak bilirubin 23.4 and 30.5 mg/dL, ALT 65 and 96 U/L, Alk P 202 and 398 U/L], resulting in death in one and emergency liver transplant in the other).
- Marchan-Lopez A, Dominguez-Dominguez L, Kessler-Saiz P, Jarrin-Estupiñan ME. Liver failure in human immunodeficiency virus - hepatitis C virus coinfection treated with sofosbuvir, ledipasvir and antiretroviral therapy. J Hepatol. 2016;64:752–3. [PubMed: 26682727](Letter in response to Dyson [2016]: 49 year old man with chronic hepatitis C, cirrhosis [Child-Pugh class B] and HIV coinfection developed worsening hepatic decompensation 1 to 2 months after starting sofosbuvir and ledipasvir that worsened for two weeks after stopping [peak bilirubin 46.9 mg/dL, INR 3.17], and then resolved; he later tolerated reinitiation of antiretroviral drugs).
- Dyson JK, McPherson S. Reply to "Liver failure in human immunodeficiency virus - Hepatitis C virus coinfection treated with sofosbuvir, ledipasvir and antiretroviral therapy". J Hepatol. 2016;64:753–4. [PubMed: 26682725](Letter in reply to March-Lopez [2016] reporting another case of hepatic decompensation during sofosbuvir, ledipasvir and ribavirin therapy of a patient hepatitis C, cirrhosis and HIV coinfection, arising within 6 weeks of starting treatment [bilirubin 12.6 mg/dL, protime 17 sec], and leading to successful, emergency liver transplantation).
- Welker MW, Luhne S, Lange CM, Vermehren J, Farnik H, Herrmann E, Welzel T, et al. Lactic acidosis in patients with hepatitis C virus cirrhosis and combined ribavirin/ sofosbuvir treatment. J Hepatol. 2016;64(4):790–9. [PubMed: 26658684](Among 35 patients with chronic hepatitis C and advanced fibrosis or cirrhosis treated with sofosbuvir based regimens, 12 [34%] had a serious adverse event and 5 [14%] developed lactic acidosis, largely in those with Child-Pugh class B or C cirrhosis and in the context of hepatic decompensation, 2 of whom died).
- Hoofnagle JH. Hepatic decompensation during direct-acting antiviral therapy of chronic hepatitis C. J Hepatol. 2016;64(4):763–5. [PubMed: 26795828](Editorial in response to Welker [2016] discussing the occurrence of unexplained hepatic decompensation during antiviral therapy of hepatitis C and whether these episodes are coincidental, caused by hepatoxicity of the antiviral drugs, or are the paradoxical result of sudden eradication of the chronic viral infection).
- Abdulsamad M, Ihimoyan A. Viekira Pak induced fatal lactic acidosis: a case report of an unusual side effect. Case Reports Hepatol. 2016;2016:8627139. [PMC free article: PMC5156790] [PubMed: 28044114](69 year old man with HCV related cirrhosis developed lactic acidosis and renal failure 3 days after starting Viekira Pak, dying within a few days of multiorgan failure).
- Masetti M, Magalotti D, Martino E, Andreone P, Scuteri A, Zoli M. A case of acute liver failure during ritonavir-boosted paritaprevir, ombitasvir and dasabuvir therapy in a patient with HCV genotype 1b cirrhosis. J Gastrointestin Liver Dis. 2016;25:559–561. [PubMed: 27981315](84 year old man with HCV-related cirrhosis developed hepatic decompensation 13 days after starting Viekira Pak for HCV infection with slow and incomplete recovery upon stopping).
- Buzas C, Tantau M, Ciobanu L. Fatal acute liver failure during ritonavir-boosted paritaprevir, ombitasvir and dasabuvir plus ribavirin therapy. J Gastrointestin Liver Dis. 2017;26:93–94. [PubMed: 28338122](65 year old woman with HCV-related cirrhosis developed hepatic decompensation 3 days after starting Viekira Pak therapy for hepatitis C and died of hepatic failure 19 days later).
- Fofiu C, Dobru D, Boeriu A. Potential pitfalls of Viekira Pak™ therapy in patients with HCV genotype 1b cirrhosis. J Gastrointestin Liver Dis. 2017;26:94–95. [PubMed: 28338123](60 year old woman with HCV related cirrhosis developed decompensation 6 weeks after starting Viekira Pak for HCV infection [bilirubin rising from 2.1 to 6.8 mg/dL, INR 1.4 to 1.8], resolving slowly after discontinuation of the combination antiviral therapy; role of ritonavir unclear).
- Oberg CL, Hiensch RJ, Poor HD. Ombitasvir-paritaprevir-ritonavir-dasabuvir (Viekira Pak)-induced lactic acidosis. Crit Care Med. 2017;45:e321–e325. [PubMed: 27661862](Three patients with HCV related cirrhosis developed severe lactic acidosis within 5-11 days of starting Viekira Pak, 2 responding to intensive care support and one dying within 36 hours with worsening lactic acidemia and shock).
- Chamorro-de-Vega E, Gimenez-Manzorro A, Rodriguez-Gonzalez CG, Escudero-Vilaplana V, De Lorenzo-Pinto A, Iglesias-Peinado I, Herranz-Alonso A, et al. GRUviC Study Group. Twelve weeks of ombitasvir/paritaprevir/r and dasabuvir without ribavirin is effective and safe in the treatment of patients with HCV genotype 1b infection and compensated cirrhosis: results from a real-world cohort study. Expert Opin Drug Saf. 2018;17:235–241. [PubMed: 29325476](Among 78 patients with chronic hepatitis C, genotype 1b, treated with Viekira Pak for 12 weeks, the SVR rate was 96% and 78% of patients had an adverse event which were mostly mild, but included 1 patient who developed hepatic decompensation at 4 weeks and stopped therapy early).
- Isakov V, Paduta D, Viani RM, Enejosa JV, Pasechnikov V, Znoyko O, Ogurtsov P, et al. Ombitasvir/paritaprevir/ ritonavir + dasabuvir+ribavirin for chronic hepatitis C virus genotype 1b-infected cirrhotics (TURQUOISE-IV). Eur J Gastroenterol Hepatol. 2018;30:1073–1076. [PubMed: 29762255](Among 36 patients with chronic hepatitis C [genotype 1b] and cirrhosis treated with Viekira Pak, the SVR rate was 100% and there were no serious adverse events or episodes of hepatic decompensation or rise of ALT above 5 times ULN).
- Ascione A, De Luca M, Melazzini M, Montilla S, Trotta MP, Petta S, Puoti M, et al. ABACUS Study Group. Safety and efficacy of ombitasvir/paritaprevir/ritonavir/dasabuvir plus ribavirin in patients over 65 years with HCV genotype 1 cirrhosis. Infection. 2018;46:607–615. [PubMed: 29808463](Among 241 Italian patients with chronic hepatitis C, genotype 1, and cirrhosis treated with Viekira Pak with or without ribavirin for 12 to 24 weeks, the SVR rate was 95% and adverse events arose in 31% including 3 patients with hepatic decompensation, two with ascites and 1 with encephalopathy all of whom recovered and had an SVR).
- Pariente A, Arpurt JP, Rémy AJ, Rosa-Hézode I, Causse X, Heluwaert F, Macaigne G, et al. Association nationale des gastroentérologues des hôpitaux (ANGH). Hepatitis C treatment with all-oral direct-acting antivirals: Effectiveness and tolerance in a multicenter, prospective, observational study from French general hospitals (APROVVIE, ANGH). Presse Med. 2019;48(3 Pt 1):e101–e110. [PubMed: 30853287](Among 1123 patients with chronic hepatitis C [half with cirrhosis] treated with various direct acting early, oral, antiviral regimens in 24 French general hospitals, the overall SVR rate was 91% and serious adverse event rate 5.6%, with two deaths due to hepatic decompensation).
- Chen CH, Chen CH, Lin CL, Lin CY, Hu TH, Tung SY, Hsieh SY, et al. Real-world safety and efficacy of paritaprevir/ritonavir/ombitasvir plus dasabuvir ± ribavirin in patients with hepatitis C virus genotype 1 and advanced hepatic fibrosis or compensated cirrhosis: a multicenter pooled analysis. Sci Rep. 2019;9:7086. [PMC free article: PMC6506536] [PubMed: 31068655](Among 898 patients with chronic hepatitis C, genotype 1, treated with Viekira Pak with or without ribavirin for 12 or 24 weeks, the overall SVR rate was 99%, 18 patients [2%] developed hepatic decompensation [usually within 1-4 weeks], 2 had ALT elevations above 10 times ULN, and 8 of 56 [14]} with preexisting HBsAg not on therapy developed reactivation, 2 with accompanying hepatitis).
- Ferenci P, Bourgeois S, Buggisch P, Norris S, Curescu M, Larrey D, Marra F, et al. Real-world safety and effectiveness of ombitasvir/paritaprevir/ritonavir ± dasabuvir ± ribavirin in hepatitis C virus genotype 1- and 4-infected patients with diverse comorbidities and comedications: A pooled analysis of post-marketing observational studies from 13 countries. J Viral Hepat. 2019;26:685–696. [PMC free article: PMC6849558] [PubMed: 30739368](Among 3850 patients with chronic hepatitis C [genotypes 1 and 4; 35% with cirrhosis] treated with Viekira Pak with or without ribavirin for 8, 12 or 24 weeks in observational studies from 13 countries, the SVR rate was 96% and adverse event rate 26%, with 3% serious adverse events including 7 cases of hepatic failure, 3 deaths from liver related causes and 17 episodes of ALT elevations above 5 times ULN).
- Poordad F, Sedghi S, Pockros PJ, Ravendhran N, Reindollar R, Lucey MR, Epstein M, et al. Efficacy and safety of ombitasvir/paritaprevir/ritonavir and dasabuvir with low-dose ribavirin in patients with chronic hepatitis C virus genotype 1a infection without cirrhosis. J Viral Hepat. 2019;26:1027–1030. [PMC free article: PMC6850388] [PubMed: 30980576](Among 105 patients with chronic hepatitis C, genotype 1a, without cirrhosis treated with Viekira Pak with low dose ribavirin [600 mg/d] for 12 weeks, the SVR rate was 90% and 73% of patients had an adverse event, 3 of which were severe; ALT elevations above 3 times ULN arose in 2 patients but none had hepatic decompensation or clinically apparent liver injury).
- Londoño MC, Riveiro-Barciela M, Ahumada A, Muñoz-Gómez R, Roget M, Devesa-Medina MJ, Serra MÁ, et al. Effectiveness, safety/tolerability of OBV/PTV/r ± DSV in patients with HCV genotype 1 or 4 with/without HIV-1 co-infection, chronic kidney disease (CKD) stage IIIb-V and dialysis in Spanish clinical practice – Vie-KinD study. PLoS One. 2019;14:e0221567. [PMC free article: PMC6759177] [PubMed: 31550267](Among 135 patients with chronic hepatitis C [genotypes 1 and 4] and renal dysfunction [78% on dialysis] treated with Viekira Pak or Technive with or without ribavirin in 31 Spanish centers, the SVR rate was 93%, but only 1 patient failed to have a virologic response; the adverse event rate was 47%, 6 patients discontinued therapy because of side effects and 3 died during therapy, one from hepatic failure).
- Manuel Sousa J, Vergara M, Pulido F, Sánchez Antolín G, Hijona L, Carnicer F, Rincón D, et al. Real-world evidence of the effectiveness of ombitasvir-paritaprevir/r ± dasabuvir ± ribavirin in patients monoinfected with chronic hepatitis C or coinfected with human immunodeficiency virus-1 in Spain. PLoS One. 2019;14:e0225061. [PMC free article: PMC6850697] [PubMed: 31714950](Among 2408 patients with chronic hepatitis C [genotypes 1 and 4] with [n=386] or without HIV infection treated with Viekira Pak with or without ribavirin for 12 or 24 weeks at 61 Spanish sites, the SVR rate was 95% in those with HIV infection and 97% in those without, while adverse events arose in 25% overall which were serious in only 2%; no mention of acute liver injury or hepatic decompensation).
- Poordad F, Castro RE, Asatryan A, Aguilar H, Cacoub P, Dieterich D, Marinho RT, et al. Long-term safety and efficacy results in hepatitis C virus genotype 1-infected patients receiving ombitasvir/paritaprevir/ritonavir and dasabuvir ± ribavirin in the TOPAZ-I and TOPAZ-II trials. J Viral Hepat. 2020;27:497–504. [PubMed: 31954087](Among 2211 patients with chronic hepatitis C genotype 1 treated successfully with Viekira Pak and enrolled in 2 long term follow up studies, SVR was maintained in more than 99% for up to 3 years and there was a decrease in mean Fib-4 scores, and mean increases in platelet count and serum albumin levels; among 354 with cirrhosis 7 patients had an episode of decompensation, 5 liver cancer, and 2 liver transplant, but only one suffered a liver related death).
- Değertekin B, Demir M, Akarca US, Kani HT, Üçbilek E, Yıldırım E, Güzelbulut F, et al. Real-world efficacy and safety of Ledipasvir + Sofosbuvir and Ombitasvir/Paritaprevir/Ritonavir ± Dasabuvir combination therapies for chronic hepatitis C: A Turkish experience. Turk J Gastroenterol. 2020;31:883–893. [PMC free article: PMC7928249] [PubMed: 33626001](Among 4352 patients with chronic hepatitis C treated with Viekira Pak or Harvoni with or without ribavirin for 12 or 24 weeks at 36 medical centers in Turkey, the SVR rate was 93% and was similar with all regimens, while adverse events were reported in 19% of patients, but there were no treatment related serious adverse events, although there were 3 liver-related deaths).
- Hung HY, Chen CY, Liao YH. A retrospective cohort study: safety and effectiveness of elbasvir/grazoprevir ± ribavirin compared with ombitasvir/paritaprevir/ritonavir/dasabuvir ± ribavirin in patients with chronic hepatitis C genotype 1 infection. Front Pharmacol. 2021;12:640317. [PMC free article: PMC8458878] [PubMed: 34566631](Among 254 patients with chronic hepatitis C, genotype 1, treated with Mavyret or Viekira Pak with or without ribavirin for 12 or 24 weeks, the SVR rate was similar in the two groups while ALT elevations above 5 times ULN arose in 4 of 105 receiving Mavyret vs 2 of 149 on Viekira Pak).
- Torgersen J, Newcomb CW, Carbonari DM, Rentsch CT, Park LS, Mezochow A, Mehta RL, et al. Protease inhibitor-based direct-acting antivirals are associated with increased risk of aminotransferase elevations but not hepatic dysfunction or decompensation. J Hepatol. 2021;75:1312–1322. [PMC free article: PMC8604762] [PubMed: 34333102](Among propensity matched subgroups of 71,391 adults with chronic hepatitis C treated in the Veterans Administration Medical system with oral, direct acting antiviral agents, patients with higher baseline fibrosis scores compared to those with lower scores were more likely to have serum ALT elevations [>200 U/L] [0.5% vs 0.3%] and hepatic decompensation [0.41% vs 0.03%], and ALT elevations were more frequent with Viekira Pak and Technive [1.1-1.2%] compared to Mavyret, Zepatier, Epclusa, and Harvoni [0.11-0.39%]).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Zepatier.[LiverTox: Clinical and Researc...]Review Zepatier.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Drug Induced Pneumonitis Secondary to Treatment with Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir (VIEKIRA PAK®) for Chronic Hepatitis C: Case Report of an Unexpected Life-Threatening Adverse Reaction.[Case Rep Med. 2017]Drug Induced Pneumonitis Secondary to Treatment with Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir (VIEKIRA PAK®) for Chronic Hepatitis C: Case Report of an Unexpected Life-Threatening Adverse Reaction.Wu SY, Faire B, Gane E. Case Rep Med. 2017; 2017:4895736. Epub 2017 Mar 20.
- Review Sofosbuvir.[LiverTox: Clinical and Researc...]Review Sofosbuvir.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review New combination antiviral for the treatment of hepatitis C.[Am J Health Syst Pharm. 2016]Review New combination antiviral for the treatment of hepatitis C.Lam JT, Salazar L. Am J Health Syst Pharm. 2016 Jul 15; 73(14):1042-50. Epub 2016 May 23.
- Viekira Pak Induced Fatal Lactic Acidosis: A Case Report of an Unusual Side Effect.[Case Reports Hepatol. 2016]Viekira Pak Induced Fatal Lactic Acidosis: A Case Report of an Unusual Side Effect.Abdulsamad M, Ihimoyan A. Case Reports Hepatol. 2016; 2016:8627139. Epub 2016 Dec 1.
- Viekira Pak - LiverToxViekira Pak - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...