NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Vilazodone is a selective serotonin reuptake inhibitor (SSRI) and partial serotonin receptor agonist which is used in the therapy of major depressive disorders. In premarketing clinical trials, vilazodone therapy was not associated with an increased rate of elevations in serum aminotransferase levels, and it has yet to be linked to instances of clinically apparent acute liver injury.
Background
Vilazodone (vil az' oh done) is an SSRI that acts by blocking the reuptake of serotonin in CNS synaptic clefts, thus increasing serotonin levels in the brain which is associated with its psychiatric effects. Vilazodone is also a partial serotonin (5-HT1A) receptor agonist, which may add to its antidepressant effects. Vilazodone was approved for use in the United States in 2011 for use in treatment of major depressive disorder. There is limited clinical experience with its use. Vilazodone is available as tablets of 10, 20 and 40 mg under the brand name Viibryd. The recommended initial dose of vilazodone in adults is 10 mg daily, which can then be increased to the typical maintenance dose of 40 mg once daily. Common, non-serious side effects include diarrhea, nausea, fatigue, drowsiness, headache, insomnia, weight gain and sexual dysfunction. Overdose is associated with acute serotonin syndrome. Rare, but potentially severe adverse effects include suicidal thinking and behavior, activation of symptoms of mania, serotonin syndrome, sexual dysfunction, hyponatremia and hypersensitivity reactions.
Hepatotoxicity
In premarketing studies, liver test abnormalities were uncommon in patients taking vilazodone (<1%) and no more frequent than in placebo recipients. No instances of acute, clinically apparent liver injury attributed to vilazodone have been reported. However, vilazodone has been in use for a short period of time. Most other SSRIs in clinical use have been associated with rare instances of acute liver injury, usually arising within 2 to 8 weeks of starting therapy. The pattern of serum enzyme elevations varied from hepatocellular to cholestatic. Autoimmune markers are not common, but immunoallergic features (rash, fever, eosinophilia) are frequent but usually not prominent. Most cases of acute liver injury due to SSRIs are mild-to-moderate in severity and resolve within one to three months. Acute liver failure due to the SSRIs has been described, but is very rare. No such cases have been linked to vilazodone use.
Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which vilazodone might cause liver injury is not known. Vilazodone is metabolized in the liver at least in part through cytochrome P450 pathways, predominantly CYP 3A4. It is susceptible to significant drug-drug interactions with increased serum levels when given with strong CYP 3A4 inhibitors (such as ketoconazole) and with reduced concentrations when given with strong inducers (such as carbamazepine).
Outcome and Management
The serum aminotransferase elevations that occur on amoxapine therapy are usually self-limited and do not require dose modification or discontinuation of therapy. No instances of acute liver failure or vanishing bile duct syndrome due to amoxapine have been reported. There is no information on cross sensitivity to liver injury between amoxapine and other tricyclic antidepressants, but switching to another class of agents (such as the selective serotonin reuptake inhibitors) is probably prudent.
Drug Class: Antidepressant Agents
Other Drugs in the Subclass, SNRIs/SSRIs: Citalopram, Escitalopram, Duloxetine, Fluoxetine, Fluvoxamine, Levomilnacipran, Paroxetine, Sertraline, Venlafaxine, Vortioxetine
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Vilazodone – Viibryd®
DRUG CLASS
Antidepressant Agents
Product labeling at DailyMed, National Library of Medicine, NIH
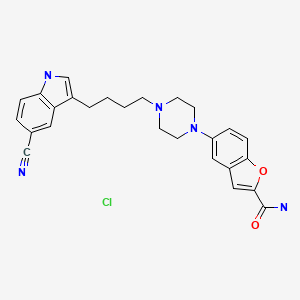
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Vilazodone | 163521-08-2 | C26-H27-N5-O2.Cl-H |
|
ANNOTATED BIBLIOGRAPHY
References updated: 08 April 2020
Abbreviations: MAO inhibitor, monoamine oxidase inhibitor; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin and norepinephrine reuptake inhibitor.
- Zimmerman HJ. Antidepressants. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 493-8.(Expert review of hepatotoxicity published in 1999; before the availability of vilazodone).
- Larrey D. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 2nd ed. New York: Informa Healthcare USA, 2007, pp. 507-26.(Review of hepatotoxicity of antidepressants published in 2007; clinically apparent liver injury from the SSRIs is rare, but probably underreported. "The clinical picture is variable, acute hepatocellular hepatitis appearing to be the most frequent event." No mention of vilazodone).
- O'Donnell JM, Bies RR, Shelton RC. Drug therapy of depression and anxiety disorders. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 267-77.(Textbook of pharmacology and therapeutics).
- Mourilhe P, Stokes PE. Risks and benefits of selective serotonin reuptake inhibitors in the treatment of depression. Drug Saf. 1998;18:57–82. [PubMed: 9466088](Review of pharmacology, efficacy and safety of SSRIs; no mention of ALT elevations or hepatotoxicity or of vilazodone).
- Lucena MI, Carvajal A, Andrade RJ, Velasco A. Antidepressant-induced hepatotoxicity. Expert Opin Drug Saf. 2003;2:249–62. [PubMed: 12904104](Review of hepatotoxicity of antidepressants; antidepressant use has increased markedly between 1992 and 2002, accounting for 5% of cases of hepatotoxicity; SSRIs are less likely to cause injury than tricyclics and MAO inhibitors; range of presentations, typically self-limited and rapid recovery; no hallmarks of hypersensitivity; no mention of vilazodone).
- Spigset O, Hä S, Bate A. Hepatic injury and pancreatitis during treatment with serotonin reuptake inhibitors: data from the World Health Organization (WHO) database of adverse drug reactions. Int Clin Psychopharmacol. 2003;18:157–61. [PubMed: 12702895](Among 27,542 reports of hepatic injury in WHO database, 786 were related to SSRIs [3%], including citalopram 42, fluoxetine 222, fluvoxamine 54, paroxetine 191, sertraline 112, nefazodone 91 and venlafaxine 74; only nefazodone has an excess of hepatic reports in relationship to total reports; no mention of vilazodone).
- Degner D, Grohmann R, Kropp S. RüE, Bender S, Engel RR, Schmidt LG. Severe adverse drug reactions of antidepressants: results of the German multicenter drug surveillance program AMSP. Pharmacopsychiatry. 2004;37 Suppl 1:S39–45. [PubMed: 15052513](Analysis of adverse drug reactions reported from 1993-2000 in 35 psychiatric hospitals; 0.7% of SSRI recipients had a severe adverse event, hepatic in 0.05%).
- Pinzani V, Peyriere H, Hillaire-Buys D, Pageaux GP, Blayac BP, Larrey D. Specific serotonin recapture inhibitor (SSRI) antidepressants: hepatotoxicity assessment in a large cohort in France. J Hepatol. 2006;44:S256.(Abstract: Analysis of French Pharmacovigilance data on SSRIs found 63 cases of hepatotoxicity from paroxetine, 45 fluoxetine, 30 citalopram, 18 sertraline, and 2 fluvoxamine; vilazodone not mentioned).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were linked to any of the SSRIs).
- Laughren TP, Gobburu J, Temple RJ, Unger EF, Bhattaram A, Dinh PV, Fossom L, et al. Vilazodone: clinical basis for the US Food and Drug Administration's approval of a new antidepressant. J Clin Psychiatry. 2011;72:1166–73. [PubMed: 21951984](FDA analysis of data on safety and efficacy of vilazodone that led to its approval; 2989 subjects were exposed to vilazodone in 32 trials; common side effects were diarrhea [28%], nausea [23%], vomiting [5%], insomnia [6%], palpitations, fatigue and sexual dysfunction; overdose was associated with acute serotonin syndrome; "vilazodone was not associated with any clear finding of drug related changes in laboratory parameters, vital signs or weight").
- Vilazodone (Viibryd)--a new antidepressant. Med Lett Drugs Ther. 2011;53(1368):53–4. [PubMed: 21738107](Concise review of mechanism of action, efficacy, safety and cost of vilazodone shortly after its approval in the US mentions that common side effects are diarrhea and nausea and occasionally insomnia, dizziness, headache, weight gain and rarely sexual dysfunction; no mention of ALT elevations or hepatotoxicity).
- Liebowitz M, Croft HA, Kajdasz DK, Whalen H, Gallipoli S, Athanasiou M, Reed CR. The safety and tolerability profile of vilazodone, a novel antidepressant for the treatment of major depressive disorder. Psychopharmacol Bull. 2011;44:15–33. [PMC free article: PMC5044546] [PubMed: 27738360](Among 1485 patients with major depression enrolled in two controlled trials of vilazodone, diarrhea, nausea and insomnia were the most frequent adverse events and there were no serum ALT elevations above 3 times ULN or serious hepatic adverse events).
- Choi E, Zmarlicka M, Ehret MJ. Vilazodone: a novel antidepressant. Am J Health Syst Pharm. 2012;69:1551–7. [PubMed: 22935937](Review of the structure, mechanism of action, pharmacology, efficacy and safety of vilazodone; in three clinical trials, ALT elevations were not mentioned or said to be no more frequent with vilazodone than placebo).
- Citrome L. Vilazodone for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2012;66:356–68. [PubMed: 22284853](Systematic review of safety and efficacy of vilazodone in depression; "Vilazodone was not associated with any clinically important changes in laboratory test parameters in serum chemistry, including liver function tests").
- Robinson DS, Kajdasz DK, Gallipoli S, Whalen H, Wamil A, Reed CR. A 1-year, open-label study assessing the safety and tolerability of vilazodone in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31:643–6. [PubMed: 21869687](Among 616 patients with depression enrolled in a 52 week open label study of vilazodone, common side effects were diarrhea and nausea, 21% of patients stopped therapy because of adverse events, and only 2 patients [0.4%] developed ALT elevations greater than 3 times ULN; no cases of clinically apparent liver injury were reported).
- Iranikhah M, Wensel TM, Thomason AR. Vilazodone for the treatment of major depressive disorder. Pharmacotherapy. 2012;32:958–65. [PubMed: 23033234](Review of the mechanism of action, pharmacokinetics, efficacy and safety of vilazodone, based largely on premarketing trials; ALT elevations and liver injury were not mentioned).
- Park SH, Ishino R. Liver injury associated with antidepressants. Curr Drug Saf. 2013;8:207–23. [PubMed: 23914755](Review of drug induced liver injury due to antidepressants including SSRIs, does not discuss vilazodone).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, one of which was attributed to venlafaxine, but none to other SSRIs or vilazodone).
- Voican CS, Corruble E, Naveau S, Perlemuter G. Antidepressant-induced liver injury: a review for clinicians. Am J Psychiatry. 2014;171:404–15. [PubMed: 24362450](Review of the frequency and clinical features of drug induced liver injury due to antidepressants; several SSRIs are discussed [sertraline, paroxetine, fluoxetine, citalopram, fluvoxamine], but not vilazodone).
- Croft HA, Pomara N, Gommoll C, Chen D, Nunez R, Mathews M. Efficacy and safety of vilazodone in major depressive disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2014;75:e1291–8. [PubMed: 25470094](Among 505 patients with major depression treated with vilazodone [40 mg/day] or placebo for 8 weeks, common side effects were nausea, diarrhea, dizziness and insomnia and changes in liver enzymes during therapy were mild and similar between groups, no patient developing both jaundice and hepatocellular enzyme elevations [>3 times ULN]).
- Gommoll C, Durgam S, Mathews M, Forero G, Nunez R, Tang X, Thase ME. A double-blind, randomized, placebo-controlled, fixed-dose phase iii study of vilazodone in patients with generalized anxiety disorder. Depress Anxiety. 2015;32:451–9. [PMC free article: PMC4676920] [PubMed: 25891440](Among 680 patients with generalized anxiety disorder treated with vilazodone [20 or 40 mg/day] or placebo for 8 weeks, common side effects were nausea, diarrhea, dizziness and fatigue and changes in liver enzymes during therapy were similar in all groups, no patient developing both jaundice and hepatocellular enzyme elevations [>3 times ULN]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 20 cases were attributed to antidepressants including 5 to SSRIs [fluoxetine, escitalopram, sertraline], but none to vilazodone).
- Gommoll C, Durgam S, Mathews M, Forero G, Nunez R, Tang X, Thase ME. A double-blind, randomized, placebo-controlled, fixed-dose phase III study of vilazodone in patients with generalized anxiety disorder. Depress Anxiety. 2015;32:451–9. [PMC free article: PMC4676920] [PubMed: 25891440](Among 680 patients with generalized anxiety disorder treated with vilazodone [20 or 40 mg] or placebo once daily for 8 weeks, adverse events associated with vilazodone included nausea, vomiting, diarrhea, dizziness and fatigue, and changes in laboratory values were “generally small and similar among groups” and there were no instances of clinically apparent liver injury).
- Mathews M, Gommoll C, Chen D, Nunez R, Khan A. Efficacy and safety of vilazodone 20 and 40 mg in major depressive disorder: a randomized, double-blind, placebo-controlled trial. Int Clin Psychopharmacol. 2015;30:67–74. [PMC free article: PMC4314105] [PubMed: 25500685](Among 1133 patients with major depression treated with vilazodone [20 or 40 mg], citalopram [40 mg] or placebo for 10 weeks, clinical improvements in depression scores were similar in the 3 treatment arms and greater than with placebo; while adverse events more frequent with vilazodone were diarrhea [26%], nausea [21-24%], and insomnia [6-7%] and rates of laboratory test abnormalities” were low and similar across treatment groups” and there were no cases of clinically apparent liver injury).
- Baldwin DS, Chrones L, Florea I, Nielsen R, Nomikos GG, Palo W, Reines E. The safety and tolerability of vortioxetine: Analysis of data from randomized placebo-controlled trials and open-label extension studies. J Psychopharmacol. 2016;30:242–52. [PMC free article: PMC4794082] [PubMed: 26864543](Pooled analysis of 11 controlled trials and 5 extension studies of vortioxetine in 5701 patients major depression found the most common adverse events was nausea [21-31%] and that it had “no effect relative to placebo on clinical laboratory parameters”).
- Voican CS, Martin S, Verstuyft C, Corruble E, Perlemuter G, Colle R. Liver function test abnormalities in depressed patients treated with antidepressants: a real-world systematic observational study in psychiatric settings. PLoS One. 2016;11:e0155234. [PMC free article: PMC4865191] [PubMed: 27171561](Among 321 psychiatric inpatients, only 116 [36%] had liver tests performed and only 18 during therapy with an antidepressant, 3 of which were suspected to have drug induced liver injury, 1 each with escitalopram, venlafaxine and amitriptyline, all without jaundice and 2 without symptoms, all 3 resolving).
- Friedrich ME, Akimova E, Huf W, Konstantinidis A, Papageorgiou K, Winkler D, Toto S, et al. Drug-induced liver injury during antidepressant treatment: results of AMSP, a drug surveillance program. Int J Neuropsychopharmacol. 2016;19:pyv126. pii. [PMC free article: PMC4851269] [PubMed: 26721950](Among 184,234 psychiatric inpatients from 80 hospitals, 149 cases [0.08%] of drug induced liver injury were reported including 22 of 70,060 [0.03%] receiving SSRIs, 71 of 50,201 [0.14%] patients treated with tricyclics and 3 of 3869 receiving MAO inhibitors [0.08%]).
- Chen VC, Lin CF, Hsieh YH, Liang HY, Huang KY, Chiu WC, Lee Y, McIntyre RS, et al. Hepatocellular carcinoma and antidepressants: a nationwide population-based study. Oncotarget. 2017;8:30464–70. [PMC free article: PMC5444756] [PubMed: 27783998](Among almost 50,000 cases of hepatocellular carcinoma registered in the Taiwan National Health Insurance Research Database, the rate of antidepressant use was lower than in approximately 250,000 matched controls from the database).
- Ferrajolo C, Scavone C, Donati M, Bortolami O, Stoppa G, Motola D, Vannacci A, et al. DILI-IT Study Group. Antidepressant-induced acute liver injury: a case-control study in an Italian inpatient population. Drug Saf. 2018;41:95–102. [PubMed: 28770534](Among 179 cases of hospitalizations for unexplained acute liver injury enrolled in an Italian prospective study between 2010 and 2014, 17 had been exposed to antidepressants the major implicated agents being citalopram [n=4], sertraline [n=3], paroxetine [n=3], tricyclics [n=2], trazodone [n=1], fluoxetine [n=1], and duloxetine [n=1]; vilazodone not mentioned).
- Billioti de Gage S, Collin C, Le-Tri T, Pariente A, Bégaud B, Verdoux H, Dray-Spira R, et al. Antidepressants and hepatotoxicity: a cohort study among 5 million individuals registered in the French National Health Insurance Database. CNS Drugs. 2018;32:673–84. [PMC free article: PMC6061298] [PubMed: 29959758](Among 5 million persons identified in a national French health insurance database who started an antidepressant between 2010 and 2015, 382 developed serious liver injury resulting in hospitalization, rates per 100,0000 persons-years being 19 for SSRIs, 22 venlafaxine, 13 duloxetine, and 33 mirtazapine).
- Durgam S, Chen C, Migliore R, Prakash C, Edwards J, Findling RL. A phase 3, double-blind, randomized, placebo-controlled study of vilazodone in adolescents with major depressive disorder. Paediatr Drugs. 2018;20:353–63. [PMC free article: PMC6028869] [PubMed: 29633166](Among 400 patients with generalized anxiety disorder treated with vilazodone or placebo for 8 weeks, there were no differences between groups in serum enzyme elevations and no instances of clinically apparent liver injury).
- Nishimura A, Aritomi Y, Sasai K, Kitagawa T, Mahableshwarkar AR. Randomized, double-blind, placebo-controlled 8-week trial of the efficacy, safety, and tolerability of 5, 10, and 20 mg/day vortioxetine in adults with major depressive disorder. Psychiatry Clin Neurosci. 2018;72:64–72. [PubMed: 28858412](Among 600 Japanese patients with major depression treated with vortioxetine [5, 10 or 20 mg] or placebo daily for 8 weeks, response rates were not significantly higher in the treated arms and side effects more common with vortioxetine included nausea, dizziness and insomnia; one patient discontinued therapy early because of abnormal liver tests, but details not provided).
- Chan HL, Chiu WC, Chen VC, Huang KY, Wang TN, Lee Y, McIntyre RS, et al. SSRIs associated with decreased risk of hepatocellular carcinoma: A population-based case-control study. Psychooncology. 2018;27:187–92. [PubMed: 28666060](Analysis of the Taiwan National Health Service Insurance Research Database identified 59,859 patients with initial diagnosis of hepatocellular carcinoma and 285,124 matched controls; SSRI use was more frequent in the controls than in the HCC cases but only in analyses adjusted for possibly confounding factors).
- Lochmann D, Richardson T. Selective serotonin reuptake inhibitors. Handb Exp Pharmacol. 2019;250:135–44. [PubMed: 30838457](Overview of the SSRIs mentioned that they have similar efficacy in treating depression and similar rates and adverse event profile which largely represents the effects of serotonin excess: nausea, diarrhea, dizziness, somnolence, insomnia, sweating, tremor anxiety, dry mouth, anxiety and restlessness; they can also cause weigh gain and sex dysfunction as well as activate mania and cause withdrawn symptoms).
- Schwasinger-Schmidt TE, Macaluso M. Other antidepressants. Handb Exp Pharmacol. 2019;250:325–55. [PubMed: 30194544](Review of mechanism of action, pharmacology, clinical efficacy and side effects of atypical antidepressants including vilazodone which is a SSRI with additional partial agonism of the serotonin receptor with no discussion of hepatic side effects).
- Pladevall-Vila M, Pottegård A, Schink T, Reutfors J, Morros R, Poblador-Plou B, Timmer A, et al. Risk of acute liver injury in agomelatine and other antidepressant users in four European countries: a cohort and nested case-control study using automated health data sources. CNS Drugs. 2019;33:383–95. [PMC free article: PMC6441103] [PubMed: 30830574](Analysis of data sources from 4 European countries identified 3.2 million persons initiating antidepressant therapy among whom there was no increased risk for acute liver injury for agomelatine compared to citalopram, an SSRI with a low rate of hepatotoxicity).
- Drugs for anxiety disorders. Med Lett Drugs Ther. 2019;61(1578):121–6. [PubMed: 31386647](Concise review of drugs for anxiety including SSRIs, SNRIs and benzodiazepines including mechanism of action, clinical efficacy, safety and costs; does not mention ALT elevations or hepatotoxicity).
- Ueberberg B, Frommberger U, Messer T, Zwanzger P, Kuhn J, Anghelescu I, Ackermann K, Assion HJ. Drug-induced liver injury (DILI) in patients with depression treated with antidepressants: a retrospective multicenter study. Pharmacopsychiatry. 2020;53:60–4. [PubMed: 31958850](Among 329 psychiatric inpatients with depression seen at 6 psychiatric centers in Germany, 17 [5%] had serum aminotransferase elevations but none had clinically apparent liver injury, most commonly implicated drugs included mirtazapine, agomelatine, citalopram and venlafaxine).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Vilazodone: a brief pharmacological and clinical review of the novel serotonin partial agonist and reuptake inhibitor.[Ther Adv Psychopharmacol. 2011]Vilazodone: a brief pharmacological and clinical review of the novel serotonin partial agonist and reuptake inhibitor.Schwartz TL, Siddiqui UA, Stahl SM. Ther Adv Psychopharmacol. 2011 Jun; 1(3):81-7.
- Other Antidepressants.[Handb Exp Pharmacol. 2019]Other Antidepressants.Schwasinger-Schmidt TE, Macaluso M. Handb Exp Pharmacol. 2019; 250:325-355.
- Review The Preclinical and Clinical Effects of Vilazodone for the Treatment of Major Depressive Disorder.[Expert Opin Drug Discov. 2016]Review The Preclinical and Clinical Effects of Vilazodone for the Treatment of Major Depressive Disorder.Sahli ZT, Banerjee P, Tarazi FI. Expert Opin Drug Discov. 2016; 11(5):515-23. Epub 2016 Mar 16.
- Neurochemical evaluation of the novel 5-HT1A receptor partial agonist/serotonin reuptake inhibitor, vilazodone.[Eur J Pharmacol. 2005]Neurochemical evaluation of the novel 5-HT1A receptor partial agonist/serotonin reuptake inhibitor, vilazodone.Hughes ZA, Starr KR, Langmead CJ, Hill M, Bartoszyk GD, Hagan JJ, Middlemiss DN, Dawson LA. Eur J Pharmacol. 2005 Mar 7; 510(1-2):49-57.
- Review Vilazodone: a 5-HT1A receptor agonist/serotonin transporter inhibitor for the treatment of affective disorders.[CNS Neurosci Ther. 2009]Review Vilazodone: a 5-HT1A receptor agonist/serotonin transporter inhibitor for the treatment of affective disorders.Dawson LA, Watson JM. CNS Neurosci Ther. 2009 Summer; 15(2):107-17.
- Vilazodone - LiverToxVilazodone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...