Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence (CC BY-NC-ND), a copy of which is available at http://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Dostarlimab (Jemperli): CADTH Reimbursement Review: Therapeutic area: Endometrial cancer [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2022 Nov.

Dostarlimab (Jemperli): CADTH Reimbursement Review: Therapeutic area: Endometrial cancer [Internet].
Show detailsPatient Input
Canadian Cancer Society
Information Gathering
The Canadian Cancer Society gathered perspectives through distributing a survey as well as accepting testimonials from patients and caregivers. We received a total of six testimonials and 22 survey responses.Of the 22 survey respondents, there were six that had taken and two that had cared for someone who has taken Dostarlimab (eight in total). The data was gathered within the time frame of October 22 – November
All patients and caregivers who contributed a testimonial had experience with Dostarlimab.
Please note that all survey questions were directed toward the patient’s experience. When a caregiver wasasked a question, the survey indicated the questions referred to the patient.
Demographic Information for Survey Respondents
Demographic information collected from the survey is displayed below. Please note that not all surveyoptions that were offered are shown within the charts as they are limited to the options respondents actually selected.
Which of the following best describes you?
There were 20 survey respondents who were patients who currently have or previously had endometrial cancer, and two respondents who indicated they are currently or were previously a caregiver for someonewith endometrial cancer.
What Province or Territory do you currently reside in?
The majority of respondents resided in Quebec (45%) and British Columbia (27%). The other 28% resided in Ontario, Alberta, Saskatchewan and Manitoba.
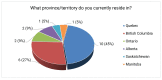
Figure 1
Geographic Location of Respondents.
How old are you?
The majority of survey respondents were between 50 and 59 and 70 and 79 years old (68%). Please refer to Figure 2 for other age brackets surveyed.

Figure 2
Age of Respondents.
What is your gender?
All respondents indicated they identify as their biological gender at birth (female), except one who self identified as male who also had experience with Dostarlimab.
Disease Experience
How much of an impact do symptoms associated with endometrial cancer have on your day-to-day activities and quality of life? (select all that apply).
The ability to conduct household chores scored highest as a day-to-day activity where patients experienceddifficulty, with ten responses in the moderate to significant impact range. The ability to travel and exercise was not far behind, with nine moderate to significant impact responses. The ability to work had the next most substantial impact, with eight responses in the moderate to significant range. There were 56 moderate to significant impact options selected across the 22 participants (34%).
Table 1
Impact of Symptoms on Day-to-Day Life and Quality of Life.
Specify any other areas of your life that have been impacted and how significant the impact is:
- “Emotional stability - significant impact”
- “I have a dance school and was unable to uphold all my duties to keep it going. Needed lots of support from family and friends. Chemo was so debilitating and this immunotherapy treatment haschanged my life. Able to live again. I hope other women can benefit from this immunotherapy instead of the huge side effects of chemo.”
- “My social life was greatly impacted. I could not go out as I wished.”
- “[The] ability to get travel insurance.”
- “Very tired lots of naps.
Experiences With Currently Available Treatments
What is the greatest financial barrier related to your treatment(s)?
The most significant financial barriers identified included travel costs and loss of income due to absencefrom work. Overall, 59% of all responders reported a financial barrier related to their treatment.
Of the four respondents who indicated travel costs were their greatest financial barrier, three had experience with Dostarlimab. Both respondents who indicated drug costs were a major barrier were also users of Dostarlimab. Additionally, three of the four respondents who selected “loss of income due to absence from work” had experience with Dostarlimab. A total of 61% of those who indicated they have financial barriers (8 people) were those who have used or cared for someone who used the drug under evaluation. These results may reflect to the increased costs affiliated with accessing this drug via a clinicaltrial (see question 1, section 5).

Figure 3
Financial Barriers to Treatment.
How is your cancer currently being treated? (select all that apply)
The majority of responders indicated they were not receiving treatment at this time (42%) or that they were undergoing immunotherapy (38%). Eight of nine responders who indicated they were undergoingimmunotherapy also stated they had experience with Dostarlimab.
One responder was doing a combination of chemotherapy, surgery, and hormone therapy while taking Anastrozole and Caelyx. The other person undergoing chemotherapy was taking Carboplatin and Paclitaxel.The responder that selected “Other” previously had surgery and declined chemotherapy and radiation.

Figure 4
Current Cancer Treatments.
How much of an impact do the following cancer treatment side effects have on your daily life?
Table 2 below depicts how impactful prevalent cancer treatment side effects were to this group. Please note that respondents could indicate “not applicable” if they did not have a specific side effect so “no
impact” means the side effect was present at some point, but to such a small degree it did not impact the person’s life. It is also pertinent to note that even if the individual indicated they are currently not receiving treatment (see Figure 4), they still experienced side effects from prior cancer treatments.
The most significant side effect impacts included changes in libido and sexual function with ten responses (45% of people) in the moderate to significant impact range and fatigue with nine responses (41% of people) in the moderate to significant impact range. There were a total of 62 responses in the moderate to significant range, leaving 22% of all responses in this range.
Table 2
Impact of Treatment Side Effects Experienced By Respondents.
If there are any other side effects caused by your current cancer treatment(s), please specify what they are and how significant their impact is on your life:
- “Skin toxicity. Significant impact”
- “Stamina - difficulty standing for more than 30 minutes or standing & Chopping food. Sleep more than normal.”
- Bladder incontinence – significant, Hot flashes - small impact
- “sécheresse de la peau (dry skin)”
- “Vision is blurry for distance like watching TV, computer screen, can't read street signs, can't make out facial features at 10 feet away. Vision goes back to normal after chemo ends.”
- “Arthritis with moderate impact.”
- “Bikini line incision for total hysterectomy and/ omentectomy seems to have caused tummy to droop a bit like a floppy breast.”
- “Arthritis”
What improvements would you like to see in new treatments that are not achieved in currently available treatments? For example: effectiveness for relieving certain symptoms or side effects, affordability, ease of use etc.
Survey respondents indicated they would like fewer side effects such as skin issues, fatigue, bladder control,stamina, vaginal bleeding after intercourse, vaginal dryness, hair loss, pain, concentration problems (chemotherapy “fog”) and arthritis. They also indicated they would like to see more drug affordability as cancer can become expensive during treatment as usual activities are interrupted.
Treatment access was also an issue for one respondent as they indicated the drugs Carboplatin and Paclitaxel were the only pharmaceutical options in their province (Manitoba). They went on to note that other provinces and parts of the world (US and Europe) have more options.
Quotes from Testimonials Outside the Survey
Many testimonials described the harsh debilitating side effects they or their family member experienced with traditional treatments as well as an overall lack of efficacy. All patients who submitted testimonials underwent several lines of conventional therapy with poor results that led to significant reductions in quality of life, multiple side effects, stress, financial distress, and lost time. Patients and caregivers described some of these conventional treatments to have a cascading effect which led to further complications.
“The first line of chemotherapy gave me very bad side effects, pain and complications. They gave me first and second line chemotherapy sessions, both had very bad side effects and were not effective.….At that point, they gave me radiation to shrink the tumour, and yet that was only for one small tumour that I had….My abdominal pain become worse and worse. The pain medications were not effective.”
“Prior to my diagnosis I was a dance teacher for 30 years and working over 30 h a week at my dance school. Dancing and teaching children was my passion and a dream come true….The treatment suggested by my medical team was frightening and I wanted to seek alternative treatments. They explained it was not a viable option and surgery was the best decision at the time. I had my uterus and ovaries removed…it was stage 1A endometrial cancer…It wasn’t long after that pain became an issue…I was told my cancer came back and I will need to do chemotherapy. I was devastated. I had to go to the hospital every single week for treatment during my 2015 summer. Every treatment felt like a truck ran over me. I was unwell and very weak. I saw my muscles deteriorating and I couldn’t teach and dance anymore. I lost my hair, my identity, my person…finance became an issue because I had to hire other dance teachers to save my business; my income was greatly reduced. Once my chemotherapy was completed, towards the end of 2015, I started to feel better…Only to be told that my cancer has come back once again. I was on another type of chemotherapy (Caelys) for the whole year of 2016…I also had multiple appointments at the hospital, because my ureters were blocked, multiple double J changes and constant pain and discomfort caused by the double J. I couldn’t sit or lie down. I slept standing up…Toward the end of 2016, the chemotherapy that I was on didn’t work anymore. My pain was excruciating, and I couldn’t bear it anymore….[they] removed the double J to alleviate my pain…I had my bladder and rectum removed and had extensive surgery…Cancer treatment didn’t seem to be efficient and I was considered a palliative candidate…As a dancer, it meant the end of my dream and my passion but I wasn’t ready to give up.”
“I had major surgery August 20, 2014, entailing a total removal of my reproductive system, 6 hours and 4 hours in recovery. Then a full course of very aggressive chemotherapy and radiation. To say that I was at my lowest is an understatement, I have never been so ill…after a 10 day stay in the hospital I had lost 40 pounds. Very weak needing constant hep with the basic necessitates of life”.
“August 26 2016 it was the beginning of 6 cycles of Taxol/Carboplatin every 3 weeks. Her last treatment was on December 2, 2016. The chemotherapy sessions were very hard on my mom. She had a lot of adverse reactions. She was hospitalized a few times. She needed a lot of blood transfusions, neutrophil injections and her chemotherapy doses were constantly adjusted. My sister remembers so vividly the moment that our mother started losing her hair. As she was brushing her hair at the hospital to make her feel better, she was slowly brushing off chunks of hair off her head. She looked frail, different, almost ghost like as time passed. Although this was expected, the emotions that this was real, and it was actually happening flooded us like a nightmare…Doctor Jardon told us that the first line of chemotherapy sessions did not work”.
“Prior to my mother’s diagnosis, she was a janitor in an office building and she loved her work…she was very involved in the community and the children loved her. She is involved with ceremonies, traditions and she teaches [Cree] customs to children…My mother would help during hunting season. She would mostly clean, cut and prepare the meat for the conservation…After my mother received chemo treatment, we had to stay at a hotel (far from the greatest) for 2 weeks before we could go back home….I remembered my mother losing her hair and having nausea. She wasn’t involved with the community like she used to and couldn’t help her family with the trapping seasons. She also had to quit her job”.
Improved Outcomes & Experience With Drug Under Review
The following data is from the eight responders that have experience using Dostarlimab or care for someone who has used it.
How did you access TBC (Dostarlimab)?
All survey responders (and patients described in testimonials) received Dostarlimab during a clinical trial.
What were the benefits and disadvantages you experienced while taking TBC (Dostarlimab) and how did they impact your life? For example, side effects, ease of use, effectiveness, cost etc.
- “ No side effects”.
- “Highly effective”.
- “The best treatment ever”.
- “This drug saved my life. I would rather deal with the side effects than not take the drug. I see no disadvantages in taking this drug”.
- Benefits...very few side effects. I have skin problems [and] thyroid issues, but it is being remedied by medications. It seems quite effective, it has not cost me anything”.
- “Effectiveness”
- “This drug saved my mother's life and every day I am grateful that she is able to receive treatment. She constantly tells me that she would rather take this drug and deal with the side effects than not take the drug at all.”
- “My Mother did not have any side effects. [The] treatment [was] easy.”
Quotes from Testimonials
“It did save my life. I told my family that I am feeling much better and here I am today after 5 years well without major side effects except my arthritis. I survived to see my two daughters getting married and the birth of my two grandchildren. I am thankful for immunotherapy, it extended my life. Even with all the side effects I have now, I would like to continue taking the treatment and I do recommend it to other patients”.
“As of today, I have received over 30 treatments. Although I have a colostomy and urostomy, I don’t feel many side effects from this current treatment [Dostarlimab]. If I have to compare this medication to the previous chemotherapies (Paclitaxel, Carboplatin, Caelyx), radiation, and the second surgery, it’s easier thank a walk in the park! When I think of the past, I wish this medication was available from the start; I would probably not have gone through this whole nightmare and surgeries…I was able to resume my life. Teaching is still an issue because of my stomas but I am much more implicated as a director of my dance studio. I have many dancing groups and we were able to win over 100 competitions”.
“I am now approaching my third year of this trial…if it were to stop, will the cancer return and will I be able to have the same treatment or will I be shoved to the side and maybe given something else that will not work for me? I hope that this drug will be approved and that many women in my situation will have the outcome I have had. I have given three years of my life for the future of a medical miracle, I only hope I will be able to enjoy my life with my grandchildren which were born in this time frame. I have also regained 40 pounds and with adjustments I am living a wonderful life”.
“She started looking better and better. Everyone who saw her told her she looked amazing. My sister got married in May 2018 and I rushed to get married in November of the same year since my mom was doing well. We went wedding dress shopping and she was able to attend all of our events. She looked amazing. We were filled with so much joy and hope. But the best news came a month before my wedding. Tyanno called my mom and told her they do not see any tumours on her scan…I hope that everyone realized how beneficial this treatment is; how many lives it can save, but most of all. How many families could have a second chance at life.”
“Having her treatment every 6 weeks allowed my mother to be present for the family and community…Since 2018,my mom resumed her regular life. From the moose to the geese-trapping season, my mother is present to help the family. She cleans, cuts and prepares the meat for conservation. She takes part in cree ceremonies and she is much more involved in our small town community. …I could never have imagined her so well while receiving cancer treatment. I am hoping this medication could be given in a center closer to our home and not in a clinical research department so far away. It would reduce the amount of resources needed for my mother to receive treatment. She continues her Dostarlimab every 6 weeks and her cancer is very well controlled.”
“This treatment gave me the opportunity to live my life relatively normal, continue to work, support my family, and gave me at least 2 years (and still counting) of time. It was less invasive than some of the chemo options. And mild side effects. I could work, live life normally. With my family. This trial changed my life. It gave me time. And, people that are living with cancer, that is what they want. TIME.”
Was TBC (Dostarlimab) easier to use than previous therapies?
All survey responders indicated that Dostarlimab was easier to use than previous therapies. Six responders explained it was easier to use because there were no side effects to minimal side effects compared to other treatments. One responder said it was easier because the treatment was every six weeks. One responder indicated they felt the short time of infusion made it easier than other therapies.
The responses above from the survey mirror the messaging received in the testimonials. Patients and caregivers expressed that side effects were reduced significantly in number and severity, which reduced suffering and improved their quality of life significantly. Some also indicated they felt they may have been able to avoid other medical interventions if this treatment had been available sooner. They also expressed Dostarlimab was more convenient, economical and accessible with the six-week infusion schedule, particularly for those living in rural areas such as a native reservation. This schedule allowed them more time to do the things that positively impactedtheir quality of life, rather than frequently being away from home and having so much precious time allocated to cancer treatment.
Anything Else?
Please provide any additional information that you feel may be useful for the evaluation of TBC (Dostarlimab).
“[I] hope it becomes available to more women so they don’t suffer like I did for so long with extensivechemo- radiation-surgery. All bad experiences. This immunotherapy is the best. Thanks to my Drs for giving this to me.”
“I was a good candidate”.
“I strongly recommend this drug. It gave my family a second chance. My mother meant the world tous and the thought of losing her was unbearable.”
Across survey responses and testimonials, patient and caregiver responses frequently echoed similar sentiments.
- Patients are asking for treatments that are more effective, but with non-debilitating side effects so theycan continue to live an acceptable quality of life with less suffrage.
- Patients would like to have more life outside of their treatment rather than occupying their time with numerous visits to the clinic.
- Patients want to achieve the longest remission possible for themselves and have more time with theirfamily.
- Patients would like more treatment options, and they want them available in all provinces for moreequitable healthcare.
Patient Group Conflict of Interest Declaration
To maintain the objectivity and credibility of the CADTH reimbursement review process, all participants in the drugreview processes must disclose any real, potential, or perceived conflicts of interest. This Patient Group Conflict ofInterest Declaration is required for participation. Declarations made do not negate or preclude the use of the patient group input. CADTH may contact your group with further questions, as needed.
Did you receive help from outside your patient group to complete this submission? If yes, please detail thehelp and who provided it.
Patients within the network of the Division of Gynecologic Oncology at McGill University Health Center participated inthe survey along with patients within the CCS network.
Did you receive help from outside your patient group to collect or analyze data used in this submission? If yes,please detail the help and who provided it.
The network of the Division of Gynecologic Oncology at McGill University Health Center provided the testimonials which were written by patients. One nurse assisted a caregiver by writing down his verbal testimonial as indicated in the testimonial itself. They also shared our survey directly with patients.
No one assisted CCS with the analysis of the survey or testimonials. CCS was the sole author of this submission.
List any companies or organizations that have provided your group with financial payment over the pasttwo years AND who may have direct or indirect interest in the drug under review.
Table 3
Conflict of Interest Declaration for Canadian Cancer Society .
GSK has not provided funds to CCS. Please let us know if you need information related to funding from other pharma companies that provide funds to CCS. To the best of our knowledge there are no existing conflicts of interest.
I hereby certify that I have the authority to disclose all relevant information with respect to any matter involving this patient group with a company, organization, or entity that may place this patient group in a real, potential, or perceived conflict of interest situation.
Patient Group: Canadian Cancer Society
Date: November 5, 2021
Clinician Input
British Columbia Cancer Provincial Gynecological Oncology Tumour Group
About the British Columbia Cancer Provincial Gynecological Oncology Tumour Group
The BC Cancer Provincial Gynecological Oncology Tumour Group is comprised of clinicians, pathologists and researchers all involved in the treatment of patients with gynecological malignancies in the province of British Columbia. Clinicians include Medical Oncologists, Radiation Oncologists, Gynecological Oncologists and General Practitioners in Oncology. The tumour group is responsible for encouraging best standards of practice, updating treatment guidelines and advocating for access to novel therapies shown to have clinical efficacy.
Information Gathering
Please describe how you gathered the information included in the submission.
The information was gathered primarily from the GARNET trial, an open label single arm study with multiple cohorts, investigating the safety/efficacy of dostarlimab. There were two parts of the study, with part 2b enrolling patients who were diagnosed with advanced endometrial cancer and who had a mismatch repair deficient (MMRd)/microsatellite instability high (MSI- H) profile. Patients received dostarlimab IV, 500 mg q3weeks for 4 doses, then 1000 mg q6 weeks until disease progression/treatment discontinuation/withdrawal.
Current Treatments
Describe the current treatment paradigm for the disease.
Focus on the Canadian context. Please include drug and non-drug treatments. Drugs without Health Canada approval for use in the management of the indication of interest may be relevant if they are routinely used in Canadian clinical practice. Are such treatments supported by clinical practice guidelines? Treatments available through special access programs are relevant. Do current treatments modify the underlying disease mechanism? Target symptoms?
Response: There is a large unmet need for women diagnosed with advanced endometrial cancer.
Approximately 7400 women were diagnosed with endometrial cancer in 2020 in Canada, making it the most common gynecological cancer afflicting Canadian women. With 1300 people dying from the disease in 2020, it is the second most lethal gynecological cancer. Women diagnosed with early stage disease have a good prognosis, however, in the setting of having recurrent or metastatic disease, median overall survival is poor, with a 10-20% predicted 5 year survival.
In the first line setting, the standard treatment for advanced/metastatic disease would be platinum based chemotherapy, typically with carboplatin and paclitaxel, response rates in the range of 50% and median progression free survival of 13 months. In certain situations, such as hormone sensitive slow growing cancers or frail patients, endocrine therapy may be used instead of chemotherapy. Radiation therapy is an option for the palliation of symptoms.
Upon progression following first line therapy, there are no effective second line treatments available to Canadian women. The usual approach is to attempt single agent cyto-toxic therapy, however response rates are low, in the range of 15-20%, and a median progression free survival of 3 months.
Recently, pembrolizumab (an immunotherapy agent) was granted Health Canada NOC/c approval for patients diagnosed with MMRd/MSI-H endometrial cancer who have progressed on prior therapy with no other reasonable options available. Although efficacy data for pembrolizumab from the Keynote 158 trial in this setting has demonstrated a response rate of 57.1%, including a complete response rate of 16.1% and long duration of response, there is no public reimbursement for this agent for endometrial cancer.
Given the above, there is an urgent need for access to novel therapeutic agents that are well tolerated and efficacious, for the treatment of patients with advanced endometrial cancer in Canada.
Treatment Goals
What are the most important goals that an ideal treatment would address?
Examples: Prolong life, delay disease progression, improve lung function, prevent the need for organ transplant, prevent infection or transmission of disease, reduce loss of cognition, reduce the severity of symptoms, minimize adverse effects, improve health-related quality of life, increase the ability to maintain employment, maintain independence, reduce burden on caregivers.
Response: The goal of treatment would be to delay progression, prevent symptoms from disease, maintain/improve quality of life, and potentially prolong life. This would all be done while trying to prevent/minimize toxicities. Click here to enter response.
Treatment Gaps (Unmet Needs)
Considering the treatment goals in Section 4, please describe goals (needs) that are not being met by currently available treatments.
Examples: Not all patients respond to available treatments. Patients become refractory to current treatment options. No treatments are available to reverse the course of disease. No treatments are available to address key outcomes. Treatments are needed that are better tolerated. Treatment are needed to improve compliance. Formulations are needed to improve convenience.
Response: Unfortunately, once patients progress beyond first line treatment in the advanced/metastatic disease setting, the treatment goals are not met with the currently available therapies, which are single agent chemotherapy agents with poor response rates. Treatments that have demonstrated efficacy with minimal toxicity are not publicly re-imbursed.
Which patients have the greatest unmet need for an intervention such as the drug under review?
Would these patients be considered a subpopulation or niche population? Describe characteristics of this patient population. Would the drug under review address the unmet need in this patient population?
Response: Prior to recent data and regulatory approvals, patients with metastatic endometrial cancer had only one effective line of treatment available to them. Approximately 30% of patients have a MMRd/MSI-H profile (which includes Lynch Syndrome patients, who account for approximately 5% of all endometrial cancer cases). Studies have demonstrated that this patient population has the greatest potential to benefit from the use of immunotherapy/immune checkpoint inhibitors.
Place in therapy
How would the drug under review fit into the current treatment paradigm?
Is there a mechanism of action that would complement other available treatments, and would it be added to other treatments? Is the drug under review the first treatment approved that will address the underlying disease process rather than being a symptomatic management therapy? Would the drug under review be used as a first-line treatment, in combination with other treatments, or as a later (or last) line of treatment? Is the drug under review expected to cause a shift in the current treatment paradigm?
Response: For patients with metastatic endometrial cancer and who are MMRd/MSI-H, upon progression of first line platinum-based chemotherapy, dostarlimab would be used as monotherapy. The GARNET trial has demonstrated that dostarlimab causes disease regression, and therefore is not only providing symptomatic management. An objective response rate of 42.3%, including 12.7% with complete response and 29.6% with partial response. The median duration of response was not reached, after a median follow-up of 11.2 months. The estimated maintenance of response was 96.4% at 6 months and 76.8% at 12 months. In addition, the most common Grade 3 treatment-related adverse events were anemia (2.9%), colitis (1.9%) and diarrhea (1.9%). Therefore, dostarlimab provides clinically meaningful anti-tumour activity with a very favourable safety profile, and not just symptomatic management. It is anticipated that dostarlimab and other immunotherapy agents will change the treatment pathway for patients with MMRd/MSI-H endometrial cancer.
Please indicate whether or not it would be appropriate to recommend that patients try other treatments before initiating treatment with the drug under review. Please provide a rationale from your perspective.
Response: In the first line setting, patients diagnosed with metastatic endometrial cancer should be offered platinum based chemotherapy. Following progression of disease, in the setting of having a MMRd/MSI-H profile, it is recommended to proceed with immune checkpoint inhibition, such as dostarlimab, given that other treatment options (i.e. single agent non-platinum chemotherapy) offer poor rates of response. If there is rapid relapse (< 12 mo) of disease upon completion of adjuvant platinum-taxane-based chemotherapy (e.g. for stage I, II, or completely resected stage III disease) re- challenge with chemotherapy is not likely to be beneficial and is associated with significant toxicity (e.g. neuropathy, alopecia, fatigue, low blood counts). For those with dMMR or MSI-H disease, it would be recommended to treat with an immune checkpoint inhibitor in this setting.
Finally, there may be patients for whom either refuse chemotherapy or for whom chemotherapy may be contra-indicated or is already known to be very poorly tolerated. Treating with an immune checkpoint inhibitor as the first line of therapy may be appropriate in such selected cases, as long as the dMMR or MSI-H status of the cancer is confirmed.
How would this drug affect the sequencing of therapies for the target condition?
If appropriate for this condition, please indicate which treatments would be given after the therapy has failed and specify whether this is a significant departure from the sequence employed in current practice. Would there be opportunity to treat patients with this same drug in a subsequent line of therapy? If so, according to what parameters?
Response: Dostarlimab would be offered to patients in the second line setting, who have progressed on platinum-based chemotherapy and who have a MMRd/MSI-H profile. This would shift the non-platinum single agent chemotherapy agents to the third line setting, examples being doxorubicin and gemcitabine.
Which patients would be best suited for treatment with the drug under review?
Which patients are most likely to respond to treatment with the drug under review? Which patients are most in need of an intervention? Would this differ based on any disease characteristics (e.g., presence or absence of certain symptoms, stage of disease)?
Response: Patients who are diagnosed with advanced endometrial cancer and who have a dMMR or MSI-H profile are most likely to respond given data from the GARNET trial investigating dostarlimab as well as other studies involving immune checkpoint inhibitors (e.g. pembrolizumab). There are no special disease characteristics that would refine this population further.
How would patients best suited for treatment with the drug under review be identified?
Examples: Clinician examination or judgement, laboratory tests (specify), diagnostic tools (specify). Is the condition challenging to diagnose in routine clinical practice? Are there any issues related to diagnosis? (e.g., tests may not be widely available, tests may be available at a cost, uncertainty in testing, unclear whether a scale is accurate or the scale may be subjective, variability in expert opinion.) Is it likely that misdiagnosis occurs in clinical practice (e.g., underdiagnosis)? Should patients who are pre-symptomatic be treated considering the mechanism of action of the drug under review?
Response: Patients would be identified after being diagnosed with endometrial cancer and undergoing biomarker testing looking for MMRd/MSI-H. This is routinely done on all patients diagnosed with endometrial cancer, and would identify eligible patients.
Which patients would be least suitable for treatment with the drug under review?
Response: At this time, patients who do not have a MMRd/MSI-H profile would not be suitable for this particular treatment.
Is it possible to identify those patients who are most likely to exhibit a response to treatment with the drug under review? If so, how would these patients be identified?
Response: As stated previously, patients diagnosed with uterine cancer are routinely tested for the presence or absence of MMRd/MSI-H. Patients with this profile have been shown to have the greatest chance of response to immune checkpoint inhibitors such as dostarlimab.
What outcomes are used to determine whether a patient is responding to treatment in clinical practice? Are the outcomes used in clinical practice aligned with the outcomes typically used in clinical trials?
Response: Patients would be monitored through regular clinical visits, physical exam, tumour markers if relevant, review of symptoms and periodic imaging with CT scan.
What would be considered a clinically meaningful response to treatment?
Examples: Reduction in the frequency or severity of symptoms (provide specifics regarding changes in frequency, severity, and so forth). Attainment of major motor milestones. Ability to perform activities of daily living. Improvement in symptoms. Stabilization (no deterioration) of symptoms. Consider the magnitude of the response to treatment. Is this likely to vary across physicians?
Response: A meaningful response would entail symptom improvement along with evidence of disease stability/response via imaging. It would be important to also see patients have an improved functional status and be able accomplish their activities of daily living.
How often should treatment response be assessed?
Response: Typically, patients will be seen/examined every 3 weeks once treatment has started, to assess tolerance and for the presence of any toxicities. This may be extended to 6 weeks after cycle 5 of treatment, when the dostarlimab treatment intervals is lengthened. Imaging is typically done every 12 weeks, or to investigate new symptoms or physical findings.
What factors should be considered when deciding to discontinue treatment?
Examples: Disease progression (specify; e.g., loss of lower limb mobility). Certain adverse events occur (specify type, frequency, and severity). Additional treatment becomes necessary (specify)
Response: Treatment discontinuation will happen in the setting of disease progression or toxicities that indicate that it is not safe to continue on with the current therapy.
What settings are appropriate for treatment with the drug under review?
Response: This treatment can be delivered in the community setting, outpatient clinics and speciality clinics/comprehensive cancer centres. There should be appropriate staff available (physician/nursing, etc) for monitoring and evaluating any potential adverse reactions.
For non-oncology drugs, is a specialist required to diagnose, treat, and monitor patients who might receive the drug under review?
Response: N/A
Additional Information
Is there any additional information you feel is pertinent to this review?
Response: As highlighted above, given that endometrial cancer is the most common gynecological cancer affecting Canadian women, there is a large unmet need for women diagnosed with advanced disease. Data for immune checkpoint inhibitors such as dostarlimab have consistently demonstrated efficacy and should become part of standard of care. In addition, the goal of researchers has been to identify biomarkers to identify patients who are most likely to benefit from therapy, and in this case the dMMR or MSI-H status allows treatment to be used in the population most likely to benefit from therapy, reducing exposure to large populations for whom the agents will have little to no benefit, thus resulting in cost savings to the system.
Canadian patients have partnered in this research, as many participated in the GARNET study. As a result, many clinicians in Canada have patients who have responded well to immune checkpoint inhibition and continue to benefit from treatment with both complete responses and unusually long periods of disease control. Among those who respond, the benefit and treatment tolerance far exceed any results observed with either standard chemotherapy or hormone based treatments.
Conflict of Interest Declarations
To maintain the objectivity and credibility of the CADTH drug review programs, all participants in the drug review processes must disclose any real, potential, or perceived conflicts of interest. This conflict of interest declaration is required for participation. Declarations made do not negate or preclude the use of the clinician group input. CADTH may contact your group with further questions, as needed. Please see the Procedures for CADTH Drug Reimbursement Reviews (section 6.3) for further details.
Did you receive help from outside your clinician group to complete this submission? If yes, please detail the help and who provided it.
No. This submission was compiled using published clinical data and local experts only.
Did you receive help from outside your clinician group to collect or analyze any information used in this submission? If yes, please detail the help and who provided it.
No.
List any companies or organizations that have provided your group with financial payment over the past two years AND who may have direct or indirect interest in the drug under review. Please note that this is required for each clinician that contributed to the input — please add more tables as needed (copy and paste). It is preferred for all declarations to be included in a single document.
Declaration for Clinician 1
Name: Aalok Kumar
Position: Medical Oncologist, BC Cancer Surrey; Provincial Systemic Gynecological Cancer Chair, BC Cancer
Date: 25-10-2021
Table 4
Conflict of Interest Declaration for British Columbia Cancer Provincial Gynecological Oncology Tumour Group Clinician 1.
Declaration for Clinician 2
Name: Anna Tinker
Position: Medical Oncologist, BC Cancer Vancouver. BC Cancer Gyne Oncology Provincial Tumour Group Chair
Date: 25/10/2021
Table 5
Conflict of Interest Declaration for British Columbia Cancer Provincial Gynecological Oncology Tumour Group Clinician 2.
Declaration for Clinician 3
Name: Susan Ellard
Position: Medical Oncologist and Department Leader, Medical Oncology, BC Cancer — Kelowna
Date: 26-10-2021
Table 6
Conflict of Interest Declaration for British Columbia Cancer Provincial Gynecological Oncology Tumour Group Clinician 3.
Declaration for Clinician 4
Name: Janice Kwon
Position: Gynecologic Oncologist; BC Gynecology Tumour Group Surgical Chair
Date: 26-10-2021
Table 7
Conflict of Interest Declaration for British Columbia Cancer Provincial Gynecological Oncology Tumour Group Clinician 4.
Declaration for Clinician 5
Name: Alannah Smrke
Position: Medical Oncologist BC Cancer Vancouver
Date: 26-10-2021
Table 8
Conflict of Interest Declaration for for British Columbia Cancer Provincial Gynecological Oncology Tumour Group Clinician 5.
McGill University Health Centre
About the McGill University Health Centre
This submission is from the doctors and nurses of Division of Gynecologic Oncology, McGill University Health Centre (MUHC). At the MUHC, patients with endometrial cancer are treated by a team consisting of three gynecologic oncologists, one medical oncologist, 4 fellows (in a Royal College of Physicians and Surgeons of Canada accredited training program), one clinical nurse specialist, one pivot nurse and eight oncology trained research nurses. We have 16 in-patient beds. We were the first team in Quebec to be awarded level IV (supra-regional) status by the Provincial Government (Programme Québécois de Cancérologie- Ministère de la Santé et des Services Sociaux) and have maintained this position since. Our research unit - Women’s Health Research Unit (WHRU) is active in investigator initiated basic and translation research supported by peer reviewed grants as well as in cooperative group (NRG) and industry sponsored clinical trials. Our websites are:
https://www.mcgill.ca/obgyn/divisions/gynecologic-oncology
Information Gathering
The information in this submission is from our experience with own patients, our research findings (we have attached 2 papers) and our knowledge of the literature. With respect to dostarlimab, we started using it in the context of the GARNET trial in June 2017 and have more than 4 years of experience with it.
Current Treatments
Describe the current treatment paradigm for the disease.
Focus on the Canadian context. Please include drug and non-drug treatments. Drugs without Health Canada approval for use in the management of the indication of interest may be relevant if they are routinely used in Canadian clinical practice. Are such treatments supported by clinical practice guidelines? Treatments available through special access programs are relevant. Do current treatments modify the underlying disease mechanism? Target symptoms?
Response: Endometrial cancer is the 4th most common cancer in Canadian women. Approximately 7,400 women are diagnosed each year and 1,300 die from the disease. Most Canadian women are diagnosed with localized disease and are cured by surgery +/- adjuvant radiotherapy/chemotherapy. If diagnosed after the disease has disseminated (stage IV), it is difficult to cure. The disturbing fact about endometrial cancer is that it is one of the few cancers in Canada and other high resource countries inwhich the age-adjusted death rates have been rising steadily by 1.9% each year from 2009. Importantly, there is an alarming doubling of incidence in women 30-49 years. Thus, it is very important that we prevent recurrences, and if recurrences do occur, use the most appropriate treatment in terms of efficacy and toxicity.
Treatment Goals
What are the most important goals that an ideal treatment would address?
Examples: Prolong life, delay disease progression, improve lung function, prevent the need for organ transplant, prevent infection or transmission of disease, reduce loss of cognition, reduce the severity of symptoms, minimize adverse effects, improve health-related quality of life, increase the ability to maintain employment, maintain independence, reduce burden on caregivers.
Response: The most important goal is to prolong good quality life. Prolonging poor quality life with interventions that are associated with significant toxicity serves very little purpose.
Treatment Gaps (Unmet Needs)
Considering the treatment goals, please describe goals (needs) that are not being met by currently available treatments.
Examples: Not all patients respond to available treatments. Patients become refractory to current treatment options. No treatments are available to reverse the course of disease. No treatments are available to address key outcomes. Treatments are needed that are better tolerated. Treatment are needed to improve compliance. Formulations are needed to improve convenience.
Response: For patients who recur with disseminated disease after the gold standard treatment of carboplatin and taxol, there is currently NO good treatment. To-date, >20 trials have investigated various chemotherapies and biologic therapies in this subset of patients; response rates achieved with chemotherapy is below 10%, median duration of response is <4 months and median overall survival less than a year. The grade ≥3 toxicity associated with these 2nds, 3rd line chemotherapies are high, exceeding 70%.
Which patients have the greatest unmet need for an intervention such as the drug under review?
Would these patients be considered a subpopulation or niche population? Describe characteristics of this patient population. Would the drug under review address the unmet need in this patient population?
Response: The drug under review, dostarlimab is an anti-PD1, which is highly effective in patients with mismatch repair deficient endometrial cancer. Only about 20% endometrial cancers - about 1800/year irecurr in Canada. The sizable proportion of these are isolated recurrences and can be treated with surgery +/- radiation +/- chemotherapy. The 5-yr survival of newly diagnosed stage IV disease is 21%, and for recurrent chemo-naïve disease it is 21%. However, recurrence after the gold standard treatment of platinum and taxol, has a dismal prognosis with a 5-year survival of 8-9%. Fortunately about 30% of these patients have tumours that have a deficient mismatch repair pathway (dMMR). These tumours are exquisitely sensitive to dostarlimab. So dostarlimab is indicated for this subpopulation – a niche group
Place In Therapy
How would the drug under review fit into the current treatment paradigm?
Is there a mechanism of action that would complement other available treatments, and would it be added to other treatments? Is the drug under review the first treatment approved that will address the underlying disease process rather than being a symptomatic management therapy? Would the drug under review be used as a first-line treatment, in combination with other treatments, or as a later (or last) line of treatment? Is the drug under review expected to cause a shift in the current treatment paradigm?
Response: For patients with dMMR tumours, which recur after platinum-based chemotherapy, dostarlimab should be offered. It is highly effective, works quickly and has minimal toxicity. We have tried pembrolizumab in these patients as we have access to pembrolizumab from our hospital pharmacy, if we made a special case for it. Pembrolizumab works in dMMR patients but much more slowly than dostarlimab and has more side effects. However, as it available to us outside of a clinical trial but dostarlimab is not, we have quite a lot of experience with pembrolizumab for dMMR recurrence. If the patient has a high burden of disease, because pembrolizumab works much more slowly, we combine it with chemotherapy to get a handle on the disease. This increases toxicity. However, even in the face of a high burden of disease, dostarlimab works alone and patients experience relief with a couple of cycles. Grade ≥3 toxicity with dostarlimab, is very low, experienced by <3% of patient. So, in our experience, dostarlimab is the most humane, fiscally prudent option for best option for treating patients with dMMR tumours, which has recured after platinum-based chemotherapy.
Please indicate whether or not it would be appropriate to recommend that patients try other treatments before initiating treatment with the drug under review. Please provide a rationale from your perspective.
Response: It is false economy to delay starting a highly effective, treatment with minimal toxicity in this niche population. Trying 2nd line chemotherapy alone in these patients is not advisable. It does not work and just causes toxicity. Patients spent most of their time in the ER and inpatient unit with symptoms from the increasing burden of cancer or side effects of the chemotherapy.
How would this drug affect the sequencing of therapies for the target condition?
If appropriate for this condition, please indicate which treatments would be given after the therapy has failed and specify whether this is a significant departure from the sequence employed in current practice. Would there be opportunity to treat patients with this same drug in a subsequent line of therapy? If so, according to what parameters?
Response: After dostarlimab has failed in patients who had failed platinum-based chemotherapy, in our hands nothing else has worked. We counsel such patients and offer palliative/supportive care. However, if patients have responded well, but the drug was interrupted for any other reason and the disease has recurred, it would be appropriate to restart, and in our experience it works again.
Which patients would be best suited for treatment with the drug under review?
Which patients are most likely to respond to treatment with the drug under review? Which patients are most in need of an intervention? Would this differ based on any disease characteristics (e.g., presence or absence of certain symptoms, stage of disease)?
Response: The patients most likely to respond are dMMR patients. However, some patients who are MMR proficient have also responded and the responses have been durable. We are not quite certain which is the best biomarker to identify the subgroup of MMR proficient patients who respond, but we think it may be evaluation of tumour mutational burden. However, this needs more study.
How would patients best suited for treatment with the drug under review be identified?
Examples: Clinician examination or judgement, laboratory tests (specify), diagnostic tools (specify). Is the condition challenging to diagnose in routine clinical practice? Are there any issues related to diagnosis? (e.g., tests may not be widely available, tests may be available at a cost, uncertainty in testing, unclear whether a scale is accurate or the scale may be subjective, variability in expert opinion.) Is it likely that misdiagnosis occurs in clinical practice (e.g., underdiagnosis)? Should patients who are pre-symptomatic be treated considering the mechanism of action of the drug under review?
Response: The gratifying aspect of this treatment is that the biomarker test for identifying dMMR patients is readily available in any tertiary care pathology laboratory. The test is inexpensive, and interpretation is standardized and within the skill set of any gynecologic pathologist.
Which patients would be least suitable for treatment with the drug under review?
Response: Only a subset of MMR proficient endometrial cancer patients who recur after platinum-based chemotherapy are likely to respond to dostarlimab. So, for such patients, we need better biomarkers.
Is it possible to identify those patients who are most likely to exhibit a response to treatment with the drug under review? If so, how would these patients be identified?
Response: The response rate of dMMR endometrial cancer patients to dostarlimab is high, between 42-70%. In patients who respond, the responses are durable with 77% of patients continuing to respond for >12 months.
What outcomes are used to determine whether a patient is responding to treatment in clinical practice? Are the outcomes used in clinical practice aligned with the outcomes typically used in clinical trials?
Response: Patients are best monitored by CT scan and symptoms. If there is disease progression in 3 cycles, we follow up in 6 weeks to ensure it is not pseudo-progression and if progression is confirmed, we discontinue the drug.
What would be considered a clinically meaningful response to treatment?
Examples: Reduction in the frequency or severity of symptoms (provide specifics regarding changes in frequency, severity, and so forth). Attainment of major motor milestones. Ability to perform activities of daily living. Improvement in symptoms. Stabilization (no deterioration) of symptoms. Consider the magnitude of the response to treatment. Is this likely to vary across physicians?
Response: Clinical symptom relief and CT scans are the best way to monitor patients. Stable disease, partial response and complete response on CT, with minimal or manageable toxicity is a good reason to continue the treatment.
How often should treatment response be assessed?
Response: Initially, the dostarlimab is best given in 500mg doses every 3 weeks for 4 cycles and then as 1000mg every 6 weeks. After the first 3 cycles, it is best to repeat the CT to assess response. If there is no progression, and the patient is doing well the CT monitoring can be spaced out to 12 weeks.
What factors should be considered when deciding to discontinue treatment?
Examples: Disease progression (specify; e.g., loss of lower limb mobility). Certain adverse events occur (specify type, frequency, and severity). Additional treatment becomes necessary (specify)
Response: The treatment should be discontinued if there is disease progression and pseudo- progression is ruled out by a repeat CT in 6 weeks.
What settings are appropriate for treatment with the drug under review?
Response: Dostarlimab has minimal toxicity, and this is its strength. So, it can be given in the community setting, outpatient clinic etc. Patients should be educated about potential toxicity and should be monitored for AEs. However, we treat pts from the cree nation which is two plane rides away from Montreal. Patients are assessed, have blood tests, and if all is well, they are given the infusion, and leave. From our experience it would be appropriate to work closely with local physicians and share responsibility and care of patients with local doctors.
For non-oncology drugs, is a specialist required to diagnose, treat, and monitor patients who might receive the drug under review?
Response: NA.
Additional Information
Is there any additional information you feel is pertinent to this review?
Response: We believe that the humane, sensible and cost effective treatment of recurrent dMMR tumour which has failed platinum based chemotherapy is to use dostarlimab as opposed is repeating chemotherapy that does not work. We have previously reported on total direct healthcare costs associated with repeated regimens of cytotoxic chemotherapy. We reported that the proportion of patients in whom total direct health care costs exceeded $79,000/patient was only 19% when the first recurrence was treated with chemotherapy. But total direct healthcare rose steeply with every subsequent recurrence and cytotoxic regimen, so that by the time we used 2 cytotoxic regimens, the direct health care costs exceeded $79,000/patient in 47% of patients. The steep rise in healthcare costs is associated with frequent inpatient admissions that these patients needed to tide them over acute symptoms. Although the population studied for the above study were ovarian cancer patients, the cytotoxic chemotherapies we use for recurrent and advanced endometrial cancer are the same as those we use for ovarian cancer, except that they are even less effective for recurrent endometrial cancer (paper attached).
Conflict of Interest Declarations
To maintain the objectivity and credibility of the CADTH drug review programs, all participants in the drug review processes must disclose any real, potential, or perceived conflicts of interest. This conflict of interest declaration is required for participation. Declarations made do not negate or preclude the use of the clinician group input. CADTH may contact your group with further questions, as needed. Please see the Procedures for CADTH Drug Reimbursement Reviews (section 6.3) for further details.
Did you receive help from outside your clinician group to complete this submission? If yes, please detail the help and who provided it.
No.
Did you receive help from outside your clinician group to collect or analyze any information used in this submission? If yes, please detail the help and who provided it.
No.
List any companies or organizations that have provided your group with financial payment over the past two years AND who may have direct or indirect interest in the drug under review. Please note that this is required for each clinician that contributed to the input — please add more tables as needed (copy and paste). It is preferred for all declarations to be included in a single document.
Declaration for Clinician 1
Name: Lucy Gilbert, MD MSc, FRCOG
Position: Director, Division of Gynecologic Oncology, McGill University Health Centre
Date: 29/10/2021
Table 9
Conflict of Interest Declaration for McGill University Health Centre Clinician 1.
Declaration for Clinician 2
Name: Xing Zeng MDCM, FRCSC
Position: Gynecologic Oncologist
Date: 29/10/2021
Table 10
Conflict of Interest Declaration for McGill University Health Centre Clinician 2.
Declaration for Clinician 3
Name: Victoria Mandilaras MDCM, FRCSC
Position: Medical Oncologist
Date: 29-10-2021
Table 11
Conflict of Interest Declaration for McGill University Health Centre Clinician 3.
Ontario Health Cancer Care Ontario’s Drug Advisory Committee
About Ontario Health Cancer Care Ontario’s Drug Advisory Committee
Please describe the purpose of your organization. Include a link to your website (if applicable).
OH-CCO’s Drug Advisory Committees provide timely evidence-based clinical and health system guidance on drug- related issues in support of CCO’s mandate, including the Provincial Drug Reimbursement Programs (PDRP) and the Systemic Treatment Program.
Information Gathering
This input was jointly discussed with the listed DAC members.
Current Treatments
Focus on the Canadian context. Please include drug and non-drug treatments. Drugs without Health Canada approval for use in the management of the indication of interest may be relevant if they are routinely used in Canadian clinical practice. Are such treatments supported by clinical practice guidelines?Treatments available through special access programs are relevant. Do current treatments modify the underlying disease mechanism? Target symptoms?
Response: For patients who failed platinum treatment, currently there’s no standard of care treatment and minimal options. Patients receiving treatment beyond 1L are likely to have chemo-resistant disease. There is a lack of data for treatment for these patients.
Treatment Goals
What are the most important goals that an ideal treatment would address?
Examples: Prolong life, delay disease progression, improve lung function, prevent the need for organ transplant, prevent infection or transmission of disease, reduce loss of cognition, reduce the severity of symptoms, minimize adverse effects, improve health-related quality of life, increase the ability to maintain employment, maintain independence, reduce burden on caregivers.
Response: Prolong life, delay disease progression, reduce severity of symptoms, improve QoL, reduce burden on caregivers, maintain independence, minimize toxicities
Treatment Gaps (Unmet Needs)
Considering the treatment goals in Section 4, please describe goals (needs) that are not being met by currently available treatments.
Examples: Not all patients respond to available treatments. Patients become refractory to current treatment options. No treatments are available to reverse the course of disease. No treatments are available to address key outcomes. Treatments are needed that are better tolerated. Treatment are needed to improve compliance. Formulations are needed to improve convenience
Response: Less than 15% treatment response and rapid progression and death. Median PFS is around 3 to 3.5 months in this population. There is no standard second-line options and no meaningful and effective treatment for these patients. Second line agent (doxorubicin) is toxic and challenging in older population.
Which patients have the greatest unmet need for an intervention such as the drug under review?
Would these patients be considered a subpopulation or niche population? Describe characteristics of this patient population. Would the drug under review address the unmet need in this patient population?
Response: Endometrial cancer population is rising in Canada – with rising incidence and mortality (Ref: Canadian Cancer Society); 30% patients will have dMMR. Historically, these patients are insufficiently studied by clinical trials and resulting in inequity in care compared to other disease sites. Additionally, these patients are not represented by advocacy groups which may result in inequitable access to treatment. Endometrial cancer is a heterogeneous disease. It’s only recently that biomarkers are identified in this disease. There is a class effect to immunotherapy in dMMR tumours that is especially important in endometrial cancer.
Place In Therapy
How would the drug under review fit into the current treatment paradigm?
Is there a mechanism of action that would complement other available treatments, and would it be added to other treatments? Is the drug under review the first treatment approved that will address the underlying disease process rather than being a symptomatic management therapy? Would the drug under review be used as a first-line treatment, in combination with other treatments, or as a later (or last) line of treatment? Is the drug under review expected to cause a shift in the current treatment paradigm?
Response: If recommended by the Expert Committee, dostarlimab will be the first targeted agent funded for endometrial cancer. It addresses a significant gap and provide the patients with an effective and meaningful treatment beyond first-line.
Please indicate whether or not it would be appropriate to recommend that patients try other treatments before initiating treatment with the drug under review. Please provide a rationale from your perspective. If so, please describe which treatments should be tried, in what order, and include a brief rationale.
Response: There are no other funded options available
How would this drug affect the sequencing of therapies for the target condition?
If appropriate for this condition, please indicate which treatments would be given after the therapy has failed and specify whether this is a significant departure from the sequence employed in current practice. Would there be opportunity to treat patients with this same drug in a subsequent line of therapy? If so, according to what parameters?
Response: This will be given after carbo-Taxol. No effect on sequencing.
Which patients would be best suited for treatment with the drug under review?
Which patients are most likely to respond to treatment with the drug under review? Which patients are most in need of an intervention? Would this differ based on any disease characteristics (e.g., presence or absence of certain symptoms, stage of disease)?
Response: As per the indication - treatment of adult patients with recurrent or advanced mismatch repair deficient (dMMR) or microsatellite instability-high (MSI-H) endometrial cancer (EC) that has progressed on or following prior treatment with a platinum containing regimen
How would patients best suited for treatment with the drug under review be identified?
Examples: Clinician examination or judgement, laboratory tests (specify), diagnostic tools (specify). Is the condition challenging to diagnose in routine clinical practice? Are there any issues related to diagnosis? (e.g., tests may not be widely available, tests may be available at a cost, uncertainty in testing, unclear whether a scale is accurate or the scale may be subjective, variability in expert opinion.) Is it likely that misdiagnosis occurs in clinical practice (e.g., underdiagnosis)? Should patients who are pre-symptomatic be treated considering the mechanism of action of the drug under review?
Response: Reflex IHC for MMR protein is standard of care for all EC and is funded in Ontario. There’s now improved understanding of the different classification of EC and predictive value of dMMR and immunotherapy response. These patients will now need effective treatment as a result of the companion diagnostics.
Which patients would be least suitable for treatment with the drug under review?
Response: Patients with contraindications to immune checkpoint inhibitor; patients with intact MMR.
Is it possible to identify those patients who are most likely to exhibit a response to treatment with the drug under review? If so, how would these patients be identified?
Response: mismatch repair deficient (dMMR) by IHC or MSI-H by PCR
What outcomes are used to determine whether a patient is responding to treatment in clinical practice? Are the outcomes used in clinical practice aligned with the outcomes typically used in clinical trials?
Response: Standard of care – physical exam and imaging; symptom surveillance
What would be considered a clinically meaningful response to treatment?
Examples: Reduction in the frequency or severity of symptoms (provide specifics regarding changes in frequency, severity, and so forth). Attainment of major motor milestones. Ability to perform activities of daily living. Improvement in symptoms. Stabilization (no deterioration) of symptoms. Consider the magnitude of the response to treatment. Is this likely to vary across physicians?
Response: Partial, complete response or stable disease. Improvement of disease symptoms. GARNET trial demonstrated some patients have durable response.
How often should treatment response be assessed?
Response: As per standard of care
What factors should be considered when deciding to discontinue treatment?
Examples: Disease progression (specify; e.g., loss of lower limb mobility). Certain adverse events occur (specify type, frequency, and severity). Additional treatment becomes necessary (specify)
Response: disease progression, adverse events, additional treatment needed; patient’s choice
What settings are appropriate for treatment with the drug under review?
Response: Outpatient clinic
For non-oncology drugs, is a specialist required to diagnose, treat, and monitor patients who might receive the drug under review?
Response: N/A
Additional Information
Is there any additional information you feel is pertinent to this review?
Response: There is a significant unmet need for these patients. Although there are other Health Canada approved drug (pembrolizumab) for this indication, but it is not currently funded.
Conflict of Interest Declarations
To maintain the objectivity and credibility of the CADTH drug review programs, all participants in the drug review processes must disclose any real, potential, or perceived conflicts of interest. This conflict of interest declaration is required for participation. Declarations made do not negate or preclude the use of the clinician group input. CADTH may contact your group with further questions, as needed. Please see the Procedures for CADTH Drug Reimbursement Reviews (section 6.3) for further details.
Did you receive help from outside your clinician group to complete this submission? If yes, please detail the help and who provided it.
OH-CCO provided secretariat support to the DAC in completing this input.
Did you receive help from outside your clinician group to collect or analyze any information used in this submission? If yes, please detail the help and who provided it.
No.
List any companies or organizations that have provided your group with financial payment over the past two years AND who may have direct or indirect interest in the drug under review. Please note that this is required for each clinician that contributed to the input — please add more tables as needed (copy and paste). It is preferred for all declarations to be included in a single document.
Declaration for Clinician 1
Name: Dr. Sarah Ferguson
Position: Ontario Cancer Lead, gynecologic oncologist
Date: 25 Oct 2021
Table 12
Conflict of Interest Declaration for OH-CCO’s Drug Advisory Committee Clinician 1.
Declaration for Clinician 2
Name: Dr. Helen MacKay
Position: Head, Division of Medical Oncology & Hematology – Sunnybrook
Date: 17 Sep 2021
Table 13
Conflict of Interest Declaration for OH-CCO’s Drug Advisory Committee Clinician 2.
Declaration for Clinician 3
Name: Dr. Stephen Welch
Position: Medical oncologist
Date: 17 Sep 2021
Table 14
Conflict of Interest Declaration for OH-CCO’s Drug Advisory Committee Clinician 3.
Declaration for Clinician 4
Name: Dr. Orit Freedman
Position: Gynecologic oncologist
Date: 17 Sep 2021
Table 15
Conflict of Interest Declaration for OH-CCO’s Drug Advisory Committee Clinician 4.
Declaration for Clinician 5
Name: Dr. Taymaa May
Position: Surgical oncologist
Date: 17 Sep 2021
Table 16
Conflict of Interest Declaration for OH-CCO’s Drug Advisory Committee Clinician 5.
Declaration for Clinician 6
Name: Dr. Julie Francis
Position: Gynecologic oncologist
Date: 17 Sep 2021
Table 17
Conflict of Interest Declaration for OH-CCO’s Drug Advisory Committee Clinician 6.
Declaration for Clinician 7
Name: Dr. Leah Jutzi
Position: Gynecologic oncologist
Date: 17 Sep 2021
Table 18
Conflict of Interest Declaration for OH-CCO’s Drug Advisory Committee Clinician 7.
Declaration for Clinician 8
Name: Dr. Josee-Lyne Ethier
Position: Medical Oncologist
Date: 23 Sep 2021
Table 19
Conflict of Interest Declaration for OH-CCO’s Drug Advisory Committee Clinician 8.
Princess Margaret Cancer Centre
About Princess Margaret Cancer Centre
The Gynecologic Cancers Disease Site Group at the Princess Margaret is a multi- and inter-disciplinary team of oncologists and allied health professionals, with a clinical focus in the management of patients diagnosed and living with gynecologic cancers. The physician team consists of medical, radiation and gynecologic oncologists, with support from radiologists and pathologists with expertise in gynecologic cancers.
Information Gathering
Peer-reviewed manuscripts of completed clinical trials and conference proceedings were reviewed to provide the information included in this submission.
Current Treatments
Describe the current treatment paradigm for the disease.
Focus on the Canadian context. Please include drug and non-drug treatments. Drugs without Health Canada approval for use in the management of the indication of interest may be relevant if they are routinely used in Canadian clinical practice. Are such treatments supported by clinical practice guidelines? Treatments available through special access programs are relevant. Do current treatments modify the underlying disease mechanism? Target symptoms?
Response: Endometrial cancer is a clinically heterogenous disease. Although the majority of patients present with early-stage, potentially curable disease, 20% of patients who present with more aggressive histology cancers have a high risk of recurrent disease that is non-curative. For these patients, systemic therapy is the mainstay of treatment, but options are limited, and responses short-lived. Standard treatment options for patients with recurrent/metastatic endometrial cancer include endocrine therapy with aromatase inhibitors and progestins, and cytotoxic chemotherapy. Unfortunately, responses are not very durable. Once patients experience disease progression on the standard 1st line platinum/taxane regimens, options are extremely limited and 2nd-line chemotherapy regimens have response rates of less than 20% and provide benefit for 3 to 4 months.
An improved understanding of the molecular background of endometrial cancer including the ability to characterize specific molecular subgroups, has defined new treatment options for patients with recurrent disease. These include molecularly targeted agents, including anti-angiogenic agents, and immunomodulatory approaches such as the immune checkpoint inhibitors. Endometrial cancer is also recognized as being a more highly immunogenic tumour, making immunotherapy strategies particularly attractive options. Defects in mismatch repair (MMR) leading to microsatellite instability (MSI) defines a patient subgroup with high mutational tumour burden (TMB) that is likely to benefit from immune checkpoint inhibitor treatments.
Across different tumour types, endometrial cancer appears to have one of the highest frequencies of MSI or MMRd, with up to 30% patients with recurrent endometrial cancer in this subgroup. A smaller percentage of patients harbour oncogenic variants in POLE, which also leads to high tumour mutational burden.
At the current time, patients with endometrial cancer can only access molecularly targeted agents or immunotherapy approaches through a clinical trial. However, with the growing body of literature supporting the use of immune checkpoint inhibitors in patients with tumours with high tumour mutational burden related to MMR-defects (MMR-d) or POLE mutation, the inability to access these agents uniformly represents a significant gap in care for Canadian patients with recurrent/advanced endometrial cancer. There is currently no patient-support or compassionate access program available, and none of the immune checkpoint inhibitors are funded through provincial programs.
Treatment Goals
What are the most important goals that an ideal treatment would address?
Examples: Prolong life, delay disease progression, improve lung function, prevent the need for organ transplant, prevent infection or transmission of disease, reduce loss of cognition, reduce the severity of symptoms, minimize adverse effects, improve health-related quality of life, increase the ability to maintain employment, maintain independence, reduce burden on caregivers.
Response: Standard 2nd-line chemotherapy options include dose-dense paclitaxel and doxorubicin. Median progression-free-survival (PFS) on these regimens is 3 to 4mths, and toxicity is not insignificant. An ideal treatment would provide more durable disease control (PFS is an acceptable surrogate of disease control), with an acceptable tolerability profile leading to no adverse effects on patients’ quality of life. Ideally, an agent should also lead to improved overall survival in comparison with an accepted standard of care.
Treatment Gaps (Unmet Needs)
Considering the treatment goals in Section 4, please describe goals (needs) that are not being met by currently available treatments.
Examples: Not all patients respond to available treatments. Patients become refractory to current treatment options. No treatments are available to reverse the course of disease. No treatments are available to address key outcomes. Treatments are needed that are better tolerated. Treatment are needed to improve compliance. Formulations are needed to improve convenience.
Response: The current 2nd line options of chemotherapy provide very limited benefit for patients and are associated with significant toxicity including fatigue, neurotoxicity and myelosuppression. The benefit of chemotherapy is even more limited in patients with low grade endometrial cancers. In the subgroup of endometrial cancer patients with high mutational tumour burden due to MMR-d (MSI-hi) or POLE-mutation, immune checkpoint inhibitors, and specifically PD-1 and PD-L1 inhibitors have been demonstrated to have response rates of 40 to 50% with durable disease control. Specifically, pembrolizumab monotherapy had an overall response rate (ORR) of 57% in 49 patients with MMR-d or MSI-hi endometrial cancer. More importantly, responses were durable, with > 90% lasting longer than 9-months, which is remarkable given approximately 50% patients had already experienced disease progression on > 2 lines of systemic therapy. This study included a total of 151 patients with a range of tumour types; 15% experienced > Grade 3 treatment-related adverse events and approximately 10% had to discontinue treatment due to treatment-related toxicity (Marabelle A et al, JCO 2019). The more recent experience with dostarlimab monotherapy in 103 patients with MMR-d endometrial cancer, 40% of whom had received 2 or more prior lines of therapy, was equally impressive, with an ORR of 44%. Notably, in a larger cohort of 316 patients evaluable for safety, treatment-related adverse events > Grade 3 occurred in < 15% and lead to discontinuation of therapy in < 5% patients (Berton D, ASCO 2021).
This data supports efficacy and tolerability of PD-1 inhibitors in patients with MMR-d or MSI-high endometrial cancer. However, at this time there is no path to access these agents apart from clinical trial participation or self-funding, leading to inequities in care that can be provided.
Which patients have the greatest unmet need for an intervention such as the drug under review?
Would these patients be considered a subpopulation or niche population? Describe characteristics of this patient population. Would the drug under review address the unmet need in this patient population?
Response: Endometrial cancer is considered an immunogenic tumour and there are multiple on-going studies evaluating different combination therapies which include immunomodulatory drugs. At this time however, it is quite clear that the subgroup of patients with tumours with high TMB related to MMR-d/MSI or POLE mutations, are a group of patients who can be predicted to have a good response to monotherapy with a PD-1 inhibitor such as dostarlimab.
Place In Therapy
How would the drug under review fit into the current treatment paradigm?
Is there a mechanism of action that would complement other available treatments, and would it be added to other treatments? Is the drug under review the first treatment approved that will address the underlying disease process rather than being a symptomatic management therapy? Would the drug under review be used as a first-line treatment, in combination with other treatments, or as a later (or last) line of treatment? Is the drug under review expected to cause a shift in the current treatment paradigm?
Response: Recent studies of immune checkpoint inhibitors in patients with MMRd endometrial cancer with disease progression after platinum/taxane based therapy has demonstrated improvements in both PFS and QoL. KN-158 evaluated efficacy of pembro in patients with MSI-hi/MMR-d tumours and included 49 patients with endometrial cancer, demonstrating a response rate (RR) of 57% which included 8 pts (16%) with complete response (CR) and 20 pts (41%) with partial response. 22 pts (9%) had to d/c treatment due to adverse events. PD-L1 inhibitors avelumab and durvalumab have had ORR of 27% and 43% respectively in patients with MMR-d tumours.
Response rates in patients with MMR-proficient tumours (MMR-p) are lower. Based on the current data, monotherapy with a PD-1 inhibitor like dostarlimab should be considered for patients with MMR-d tumours who have had disease progression after 1st line platinum-based therapy.
Please indicate whether or not it would be appropriate to recommend that patients try other treatments before initiating treatment with the drug under review. Please provide a rationale from your perspective.
Response: Dostarlimab has demonstrated efficacy in patients who have had disease progression on at least one line of systemic therapy, with approximately 40% patients having received 2 or more prior lines of therapy. Based on this data, the recommendation at this time would be for patients to be considered for standard 1st line therapy with a platinum/taxane combination. At the time of disease progression, provided patients maintain good performance status (ECOG 0/1) and preserved end-organ function, the current body of evidence supports the use of PD-1 monotherapy such as dostarlimab.
Studies are on-going evaluating the benefits of dostarlimab and other immune checkpoint inhibitors in the first-line setting (i.e. in combination with chemotherapy), and therefore this strategy would only be recommended within the confines of a clinical trial.
How would this drug affect the sequencing of therapies for the target condition?
If appropriate for this condition, please indicate which treatments would be given after the therapy has failed and specify whether this is a significant departure from the sequence employed in current practice. Would there be opportunity to treat patients with this same drug in a subsequent line of therapy? If so, according to what parameters?
Response: After progression on dostarlimab patients can be considered for standard of care chemotherapy or clinical trials. At this time, there is limited data to support the use of dostarlimab-combination therapy after progression on dostarlimab. This would not be recommended outside of the confines of a clinical trial.
Which patients would be best suited for treatment with the drug under review?
Which patients are most likely to respond to treatment with the drug under review? Which patients are most in need of an intervention? Would this differ based on any disease characteristics (e.g., presence or absence of certain symptoms, stage of disease)?
Response: N/A
How would patients best suited for treatment with the drug under review be identified?
Examples: Clinician examination or judgement, laboratory tests (specify), diagnostic tools (specify). Is the condition challenging to diagnose in routine clinical practice? Are there any issues related to diagnosis? (e.g., tests may not be widely available, tests may be available at a cost, uncertainty in testing, unclear whether a scale is accurate or the scale may be subjective, variability in expert opinion.) Is it likely that misdiagnosis occurs in clinical practice (e.g., underdiagnosis)? Should patients who are pre-symptomatic be treated considering the mechanism of action of the drug under review?
Response: The most relevant selection strategy would be to identify patients with MMR-d/MSI-high tumours, and with tumours harbouring POLE mutation. MMR IHC is a common and fairly standard histological test that can be easily done on request for patients with recurrent endometrial cancer. Otherwise, patients may also be identified if they have had had somatic molecular profiling through a translational research program.
Which patients would be least suitable for treatment with the drug under review?
Response: The current body of data suggests that patients with MMR-proficient endometrial cancer (i.e. without defects in MMR pathway) are much less likely to benefit from monotherapy with an iCPI. Recently completed and on-going studies suggest that certain subsets of these patients may benefit from approaches that combine an iCPI with another molecularly targeted agent (eg anti- angiogenic or PARP inhibitor) and therefore, this group of patients should be considered for other therapeutic strategies including clinical trials.
Is it possible to identify those patients who are most likely to exhibit a response to treatment with the drug under review? If so, how would these patients be identified?
Response: Patients can be identified by tumour MMR immunohistochemistry, MSI PCR or mutation testing for POLE (if available). PD-L1 status does not appear to predict for tumour response and would not be required
What outcomes are used to determine whether a patient is responding to treatment in clinical practice? Are the outcomes used in clinical practice aligned with the outcomes typically used in clinical trials?
Response: Response to immune checkpoint inhibitor therapy would be based on tumour assessment by CT or MR completed every 2 to 3 cycles of therapy (i.e. every 6 to 9 wks)
What would be considered a clinically meaningful response to treatment?
Examples: Reduction in the frequency or severity of symptoms (provide specifics regarding changes in frequency, severity, and so forth). Attainment of major motor milestones. Ability to perform activities of daily living. Improvement in symptoms. Stabilization (no deterioration) of symptoms.
Consider the magnitude of the response to treatment. Is this likely to vary across physicians?
Response: A clinically meaningful response would be maintaining radiographic disease control (i.e. tumour response or stabilization on CT/MR) with good tolerance of treatment (i.e. </= grade 2 treatment- related adverse effects) and stable or improving symptoms of disease. Assessment of radiographic response is objective, however determination of clinical benefit will have an element of subjectivity.
How often should treatment response be assessed?
Response: Treatment response should be assessed radiographically with computed tomography (CT) or magnetic resonance imaging (MR) every 2 to 3 cycles of therapy (ie every 6 to 9 weeks).
What factors should be considered when deciding to discontinue treatment?
Examples: Disease progression (specify; e.g., loss of lower limb mobility). Certain adverse events occur (specify type, frequency, and severity). Additional treatment becomes necessary (specify).
Disease Response: Patients would continue on treatment as long as there was a confirmed favourable response to therapy which includes a complete/partial tumour response or disease stabilization, with stable or improved symptoms and good tolerability. There are patients who may have improved or stable symptoms but evidence of tumour progression, with increase of existing or development of new lesions. This may be consistent with the phenomena of pseudo- progression. If patients are clinically stable then they would continue on treatment, If next set of imaging demonstrates progression patients should discontinue therapy.
Adverse Events: Treatment should be held for moderate-severe immune-related toxicity and managed as per standard guidelines (Haanen J, Carbonnel F, Robert C et al. Annals of Oncology 2017; 28 (4)). Re-challenge would be per discretion of treating physician in conversation with patient, and usually can be considered if severity of immune-related event was < Grade 3. (Dolladille C et al; JAMA Oncology 2020)
What settings are appropriate for treatment with the drug under review?
Response: Patients being managed at a cancer centre by oncologists with expertise in (1) systemic therapy for gynecologic cancers and (2) managing immune-related adverse events.
For non-oncology drugs, is a specialist required to diagnose, treat, and monitor patients who might receive the drug under review?
Response: N/A
References
Green A, Feinberg J and Makker V. A Review of Immune Checkpoint Blockade Therapy in Endometrial Cancer. ASCO Educational Book 40, 2020
Haanen J, Carbonnel F, Robert C et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2017; 28(4): 119-42
Dolladile C, Ederhy S, Sassier M et al. Immune Checkpoint Inhibitor Rechallenge after Immune-related Adverse Events in Patients with Cancer. JAMA Oncology 2020; 6(6): 865-871
Marabelle A, Le D, Ascierto P et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. JCO 2019; 38: 1-10
Berton D, Banerjee S, Curigliano G et al. Antitumour Activity of Dostarlimab in Patients with Mismatch Repair-Deficient (dMMR) Tumours: A Combined Analysis of 2 Cohorts in the GARNET study. 2021 ASCO Jun 4-8; Abst #2564
Conflict of Interest Declarations
To maintain the objectivity and credibility of the CADTH drug review programs, all participants in the drug review processes must disclose any real, potential, or perceived conflicts of interest. This conflict of interest declaration is required for participation. Declarations made do not negate or preclude the use of the clinician group input. CADTH may contact your group with further questions, as needed. Please see the Procedures for CADTH Drug Reimbursement Reviews (section 6.3) for further details.
Did you receive help from outside your clinician group to complete this submission? If yes, please detail the help and who provided it.
GSK team provided slides from the GARNET study presented at ASCO 2021.
Did you receive help from outside your clinician group to collect or analyze any information used in this submission? If yes, please detail the help and who provided it.
No.
List any companies or organizations that have provided your group with financial payment over the past two years AND who may have direct or indirect interest in the drug under review. Please note that this is required for each clinician that contributed to the input — please add more tables as needed (copy and paste). It is preferred for all declarations to be included in a single document.
Declaration for Clinician 1
Name: Neesha Dhani
Position: Staff Medical Oncologist, Gynecologic Cancers Disease Site Group
Date: Oct 26, 2021
Table 20
Conflict of Interest Declaration for Princess Margaret Cancer Centre Clinician 1.
Declaration for Clinician 2
Name: Amit Oza
Position: Head, Division of Medical Oncology & Hematology, Staff Medical Oncologist, Gynecologic Cancers Disease Site Group
Date: 28-Oct-2021
Table 21
Conflict of Interest Declaration for Princess Margaret Cancer Centre Clinician 2.
Declaration for Clinician 3
Name: Stephanie Lheureux
Position: Staff Medical Oncologist, Gynecologic Cancers Disease Site Group
Date: Oct 28-2021
Table 22
Conflict of Interest Declaration for Princess Margaret Cancer Centre Clinician 3.
Declaration for Clinician 4
Name: Robert Grant
Position: Staff Medical Oncologist, Gastrointestinal & Gynecologic Cancers Disease Site Groups
Date: Oct 28-2021
Table 23
Conflict of Interest Declaration for Princess Margaret Cancer Centre Clinician 4.
Saskatchewan Cancer Agency Gynecological Oncologists
About Saskatchewan Cancer Agency Gynecological Oncologists
There are a total of six Gynecological Oncologists (GO’s) in the province covering 2 sites which is the Saskatchewan Cancer Agency (SCA). Regina and Saskatoon each have 3 practitioners.
Information Gathering
We, as a group have a deep interest in having treatment options for recurrent endometrial cancer as it is so common and minimal treatment options. There are PDL-1 available in USA and a collegue used to work in USA, and so we know the pembro literature well. Subsequently the GARNET study became available. We did reach out to GSK to know more and had meeting with them.
Current Treatments
Response: After the GARNET study was reported this become clear that a level 3 study will not be performed. The FDA has approved similar agents and states and the efficacy and safety profile are very similar. My colleague who worked in the states for 4 years has experience with a similar PD 1 immune therapy with excellent result and minimal toxicity. This study supports that Dostarlimab has very similar outcomes and should be considered as having more competition means lowered costs for the HC system.
Candida needs access to this drug as well. Especially based the data, this year volume of these patient and the fact that is been used in the United States again my patient has been accessing a similar drug through an outside source thing age cost and it will impact on lifestyle probably forever.
Treatment Goals
What are the most important goals that an ideal treatment would address?
Response: Quality of life is always most important indicator in research studies for cancer especially in recurrence disease. I certainly a.m. Of the mind that if you have a palliative patient a treatment that is improving there symptoms I rely last on the imaging and her tumour markers if relevant.
Prolonging life for me as an oncologist is not the main goal although for some patients it is. In my recent at anecdotal N=1 I have a patient with the biggest oligometastasis in her chest that I have ever seen. This is refractory to hormone therapy and chemotherapy and her level of pain was almost ready to be admitted to a palliative care unit for thoracic tap block. As we do not have access to a PD-L targeted agent she is paying out of pocket and within 2 weeks of her 1st treatment she was out at the beach. This is so incredibly meaningful as a care provider but all of the examples above certainly commented the discussion. Just for some page the goals and expectations are different but this has separate control of targeting many of these areas as it is quite well tolerated
Treatment Gaps (Unmet Needs)
Considering the treatment goals in Section 4, please describe goals (needs) that are not being met by currently available treatments.
Response: I think all of these apply. Many endometrial cancer is chemo refractory because there slow growing. If they recur the will eventually become refractory to current treatment and some patients cannot tolerate doublet chemotherapy which is certainly the standard of care Sami progress sooner.
Immunotherapy also with better target agent that intuitively makes sense to use especially we have the knowledge of the MMR status and standard care. As such the targeted component of history is why should be considered for implantation into level 3 evidence even without level 3 evidence specific to this particular drug
Which patients have the greatest unmet need for an intervention such as the drug under review?
Response: Endometrial cancer is the common gynecological malignancy and rate going up steadily over the last 30 years. This trend will continue the increase of obese continues to rise. Currently we have no robust treatments when these patients progress/recur. There are new novel therapies for much rarer cancers and is a continue frustration of myself my colleagues we have no meaningful treatment options for such a large component of her patient population.
Place In Therapy
How would the drug under review fit into the current treatment paradigm?
Response: Similar drugs have been approved. There response as already chief to the paradigm in the United States. Patient's her paying out of pocket her seeing dramatic FX and make since is now weighs mainstay of cancer treatment to look at immunotherapy and more targeted agents which this is looking at MMR deficient cancers. So no this is not the 1st treatment that would be approved to address the underlying disease process however it study shows similar responses and should be an option for practitioners.
I do not see that in the near future to be considered first-line treatment based on the study profile but certainly there is some people that believe should be first-line treatment. There is some evidence that adding another PDL inhibitor to Lenvatinib has activity and another drug is being studied currently with carboplatin and paclitaxel. The treatment X of the stroke are high enough that it would give some prolonged disease-free progression however there is certainly a possibility that for some patients would not be there last line of treatment
Please indicate whether or not it would be appropriate to recommend that patients try other treatments before initiating treatment with the drug under review. Please provide a rationale from your perspective.
Response: Yes, this treatment would be provided to patients after first line, platinum containing drugs at the time of recurrence. There are more studies on another PDL-1 inhibitor and dostarlimab
How would this drug affect the sequencing of therapies for the target condition?
Response: At the current time this would be after first-line treatment. This drug would not be used again further treatment regimen many evidence that I am aware of.
Which patients would be best suited for treatment with the drug under review?
Response: Those that are DNA mismatch repair deficient/and high levels of MSI-H instability. This testing is being done on all colorectal and endometrial cancers and, as such, if we have the test already being we should be able to offer targeted agents based on this testing. It is being done anyway as it will make treatment even more cost effective because they will be more targeted and appropriate although it is not standard of care, tumour mutational burden (TMB) may also help target who is going to respond better.
Some of these patients did respond to hormone therapy and sometimes this can cause a very sustained response to treatment this to no avail standard chemotherapy often has minimal benefit.
Patients with recurrence disease all want treatment options, especially if the performance status allows an for such a common cancer does not ongoing for straight the that we have such minimal options for these patients.
I do not foresee that recommendations for treatment would be different based on disease characteristics. As there is some risk of pneumonitis with her people would be hesitant to give a patient with a significant disease burden in the chest this drug, I am not sure, but we do need options.
How would patients best suited for treatment with the drug under review be identified?
Response: Recurrent disease (measurable). Platinum given and considered resistant and no more than 2 treatments prior. MLH deficient. PDL-1 naïve. No contraindications.
Which patients would be least suitable for treatment with the drug under review?
Response: Anything poor performance status would be considered. MMR proficient be change with the use it with another drug over time, and some would be hesitant within exist pulmonary condition.
Is it possible to identify those patients who are most likely to exhibit a response to treatment with the drug under review?
Response: This would not actually change at all from current practice because all of her endometrial cancers are determined to be MMR deficient her proficient at the time of their biopsy or hysterectomy if not done prior.
Is there some other tests such is mutation burden that may be of help but these are not currently in practice routinely. What outcomes are used to determine whether a patient is responding to treatment in clinical practice?
What outcomes are used to determine whether a patient is responding to treatment in clinical practice?
Response: Although not sometimes completely documented in imaging outside of a study protocol RECIST criteria would be the standard but again in recurrence setting with palliative intent treatment this should be equally weighed with symptoms improvement if the patient is initiated on treatment with symptomatic progression. There are no tumour markers to follow this particular cancer so symptoms, radiographic imaging plus or minus exam which is often less help this particular cancer be utilized.
Certainly when disease is in the chest & symptomatic lymphadenopathy in the retroperitoneal can be easy to follow-up
What would be considered a clinically meaningful response to treatment?
Response: I think there is variability b/t all physicians but I certainly hope and believe that, as stated above physicians will look at the whole patient including imaged and patient symptom profile. ECOG this assessed at every visit and quality of life should be part of any drug implementation and I believe this drug has demonstrated this as well as its other drugs in its class for tolerability and maintain patient's quality of life How often should treatment response be assessed?
How often should treatment response be assessed?
Response: Symptom based for some but at onset of treatment likely at 3 -6 month intervals imaging would be utilized and then likely less often thereafter. Laboratory values as well as respiratory/ pulmonary status will also be assessed to ensure that treatment response is occurring without toxicity.
Sometimes a challenge with this particular drug classes that disease is often in the lung and there can be symptomatic pneumonitis with this drug which can sometimes be challenging to delineate the cause.
What factors should be considered when deciding to discontinue treatment?
Response: Measurable or clinical progression.
Toxicity: anemia that is profound transfusion, patient intolerability for non-safety related reasons such as fatigue, nausea, diarrhea, pruritus. Pneumonitis, renal failure, hyponatremia
What settings are appropriate for treatment with the drug under review?
Response: Immediate reactions are rare and so the cancer agency may allow in Community outreach centers
For non-oncology drugs, is a specialist required to diagnose, treat, and monitor patients who might receive the drug under review?
Response: N/A
Additional Information
Is there any additional information you feel is pertinent to this review?
Response: The lack of level 3 evidence should not be a penalty for this drug as the class of drug has proven its efficacy in this disease site and its parameters for use. Endometrial cancer is COMMON and lacks robust treatment options in recurrent setting and it should be a priority for Health Canada to approve appropriate options as it affect thousands of families annual. Its efficacy, tolerability, and lack of severe toxicity should be of tremendous excitement for moving through approval process easily and in EXPEDITIOUSLY. Other drugs for rarer tumours and less robust/significant benefit have approval and it is time for this common cancer treatment to be of equal importance.
We implore the thorough assessment and for approval of Dostarlimab.
Conflict of Interest Declarations
To maintain the objectivity and credibility of the CADTH drug review programs, all participants in the drug review processes must disclose any real, potential, or perceived conflicts of interest. This conflict of interest declaration is required for participation. Declarations made do not negate or preclude the use of the clinician group input. CADTH may contact your group with further questions, as needed. Please see the Procedures for CADTH Drug Reimbursement Reviews (section 6.3) for further details.
Did you receive help from outside your clinician group to complete this submission? If yes, please detail the help and who provided it.
Having a new colleague who worked in the State we are very keen to have immunotherapy access in Canada. She is constantly searching the literature and supplies with information. In gaining information we did have an academic presentation from GSK with respect to this drug at our request.
Did you receive help from outside your clinician group to collect or analyze any information used in this submission? If yes, please detail the help and who provided it.
No, we did not.
List any companies or organizations that have provided your group with financial payment over the past two years AND who may have direct or indirect interest in the drug under review. Please note that this is required for each clinician that contributed to the input — please add more tables as needed (copy and paste). It is preferred for all declarations to be included in a single document.
Declaration for Clinician 1
Name: Vickie J martin
Position: Gynecological Oncologist
Date: 20/10/2021
Table 24
Conflict of Interest Declaration for Saskatchewan Cancer Agency Gynecological Oncologists Clinician 1.
Declaration for Clinician 2
Name: Dr Laura Hopkins
Position: Gynecological Oncologists
Table 25
Conflict of Interest Declaration for Saskatchewan Cancer Agency Gynecological Oncologists Clinician 2.
The Society of Gynecologic Oncology of Canada
About the Society of Gynecologic Oncology of Canada
The Society of Gynecologic Oncology of Canada (GOC) is a non-profit multidisciplinary organization. It is the national society representing health care professionals including physicians, nurses, and scientists Involved in the treatment and prevention of gynecologic cancer. GOC strives to improve the care of women with, or who are at risk of, gynecologic cancer by raising standards of practice, encouraging ongoing research, promoting innovation in prevention, care and discovery and advancing awareness.
Information Gathering
The data for this submission is from the GARNET trial. This multi-center, open label, single-arm, multi-cohort study evaluated the safety and efficacy of dostarlimab monotherapy in 2 parts, dose escalation and expansion. Part 2B enrolled patients into 4 expansion cohorts, including a cohort with mismatch repair deficient (MMRd)/Microsatellite- instability high (MSI-H) endometrial cancers (cohort A1). The trial assessed the antitumour activity and safety of dostarlimab. Patients received dostarlimab intravenously at 500 mg every 3 weeks for 4 doses, then 1000 mg every 6 weeks until disease progression, treatment discontinuation, or withdrawal.
Current Treatments
Describe the current treatment paradigm for the disease.
Focus on the Canadian context. Please include drug and non-drug treatments. Drugs without Health Canada approval for use in the management of the indication of interest may be relevant if they are routinely used in Canadian clinical practice. Are such treatments supported by clinical practice guidelines? Treatments available through special access programs are relevant. Do current treatments modify the underlying disease mechanism? Target symptoms?
Response: Treatment options for women diagnosed with endometrial cancer represent a critical unmet need. Endometrial cancer is the most common cancer gynecologic cancer affecting women in Canada with an estimated 7400 women diagnosed in 2020. It’s the second most lethal gynecologic cancer, approximately 1300 will die of the disease in Canada in 2020. Whilst prognosis remains good for those diagnosed with early-stage disease, for those with recurrent or metastatic endometrial cancer median overall survival is short. The standard of care first-line treatment for advanced or metastatic disease is platinum-based chemotherapy, or less commonly, endocrine therapy for hormone sensitive slow growing cancers.
Radiation therapy can be used but is only applied to the palliation of cancer related symptoms and in a focal manner.
There are no effective second-line treatments accessible for Canadian women. Response rates to second-line cytotoxic agents are low (< 20%) with median Progression Free Survival (mPFS) of 3-4 months. Health Canada recently issued a NOC/c approval for immunotherapy agent pembrolizumab for dMMR or MSI-H endometrial cancers that have progressed following prior therapy and that have no satisfactory alternative treatment options. Pembrolizumab in dMMR endometrial cancer has a high response rate (57.1% ORR) a notable complete response rate (16.1% CR) and long durations of response (DOR not reached: at 12 months, 89% of patients were still in response) (phase 2 data, Keynote 158 trial). However, there is no funded access (e.g., no public funding, no Special Access Programs and no Compassionate Access) to pembrolizumab for dMMR or MSI-H endometrial cancer in Canada. Only those with insurance coverage or the capacity to self-pay have access to pembrolizumab, creating a major disparity in access to treatment. Broadly, Canadian women with dMMR or MSI-H endometrial cancer do not have access to immunotherapy.
Hormone therapy is accessible for a subset of endometrial cancers, typically those with slower growing disease that is low grade (FIGO grade 1 or 2) and ER/PR positive. Such treatments are available across Canada as they are inexpensive and funded by most jurisdictions, however, they are not highly effective (RR <40%, PFS ~3 mo) thus most women experience disease progression and require additional therapy.
Access to new, well-tolerated, therapeutic options for Canadian women diagnosed with endometrial cancer is urgently needed. GOC is advocating on behalf of its membership and Canadian women diagnosed with endometrial cancer who do not have a national advocacy organization to represent them.
Treatment Goals
What are the most important goals that an ideal treatment would address?
Examples: Prolong life, delay disease progression, improve lung function, prevent the need for organ transplant, prevent infection or transmission of disease, reduce loss of cognition, reduce the severity of symptoms, minimize adverse effects, improve health-related quality of life, increase the ability to maintain employment, maintain independence, reduce burden on caregivers.
Response: As with most oncology treatment regimens in the recurrent setting, goals are to delay progression, reduce disease burden and symptoms from the cancer, improve quality of life, and if, possible prolong life. The recurrent endometrial cancer setting, primary goals would be to improve progression free survival while minimizing toxicities and side effects from treatments.
Treatment Gaps (Unmet Needs)
Considering the treatment goals in Section 4, please describe goals (needs) that are not being met by currently available treatments. Examples: Not all patients respond to available treatments. Patients become refractory to current treatment options. No treatments are available to reverse the course of disease. No treatments are available to address key outcomes. Treatments are needed that are better tolerated. Treatment are needed to improve compliance. Formulations are needed to improve convenience.
Response: Presently, none of the treatment goals in Section 4 are being met in patients who have already received standard first-line therapy. All women with recurrent endometrial cancer become refractory to standard therapy and there is both a lack of novel treatment and limited/no access to any proven treatments.
Which patients have the greatest unmet need for an intervention such as the drug under review?
Would these patients be considered a subpopulation or niche population? Describe characteristics of this patient population. Would the drug under review address the unmet need in this patient population?
Response: Until recently, there has only been one-line of treatment for metastatic endometrial cancer. Therefore, there is a need in the entire population of those with recurrent disease. However, up to 30% of recurrent endometrial cancers are MSI-H or dMMR. This includes ~5% with endometrial cancers related to Lynch Syndrome, as hereditary cancer syndrome which increases the risk of endometrial cancers (as well as other cancers such as colorectal carcinoma). This biomarker defined population has the greatest potential to benefit from immune checkpoint inhibitor therapy.
Place In Therapy
How would the drug under review fit into the current treatment paradigm?
Is there a mechanism of action that would complement other available treatments, and would it be added to other treatments? Is the drug under review the first treatment approved that will address the underlying disease process rather than being a symptomatic management therapy? Would the drug under review be used as a first-line treatment, in combination with other treatments, or as a later (or last) line of treatment? Is the drug under review expected to cause a shift in the current treatment paradigm?
Response: Dostarlimab would be used as monotherapy in the advanced or metastatic endometrial cancers that are dMMR or MSI-H who progressed on or after prior platinum treatment. The drug would be used in biomarker defined population that is most likely to benefit from treatment. The drug causes disease regression and therefore does directly affect the underlying disease process. This agent, and others in this class, are expected to shift the paradigm in the treatment of dMMR disease, including dMMR endometrial cancer.
Please indicate whether or not it would be appropriate to recommend that patients try other treatments before initiating treatment with the drug under review. Please provide a rationale from your perspective.
Response: Patients with recurrent metastatic endometrial cancer should receive platinum-based therapy first. When those with dMMR tumours have progression following first-line platinum-based, we would recommend proceeding with treatment using a with an immune checkpoint inhibitor such as dostarlimab. Other treatment options have poor response rates and short durations of benefit, (RR <30%, mPFS < 3 mo).
How would this drug affect the sequencing of therapies for the target condition?
If appropriate for this condition, please indicate which treatments would be given after the therapy has failed and specify whether this is a significant departure from the sequence employed in current practice. Would there be opportunity to treat patients with this same drug in a subsequent line of therapy? If so, according to what parameters?
Response: Dostarlimab would be offered after progression on platinum-based therapy (in the dMMR cohort only). After progression on dostarlimab, 3rd line agents such as adriamycin, paclitaxel, gemcitabine, could be offered.
Which patients would be best suited for treatment with the drug under review?
Which patients are most likely to respond to treatment with the drug under review? Which patients are most in need of an intervention? Would this differ based on any disease characteristics (e.g., presence or absence of certain symptoms, stage of disease)?
Response: Patients with recurrent dMMR or MSI-H endometrial cancer having progressed on platinum-based therapy. As previously mentioned, this is a niche population of patients who stand to benefit the most from dostarlimab treatment, as current second line therapies have poor response rates a lower duration of responses.
How would patients best suited for treatment with the drug under review be identified?
Examples: Clinician examination or judgement, laboratory tests (specify), diagnostic tools (specify). Is the condition challenging to diagnose in routine clinical practice? Are there any issues related to diagnosis? (e.g., tests may not be widely available, tests may be available at a cost, uncertainty in testing, unclear whether a scale is accurate or the scale may be subjective, variability in expert opinion.). Is it likely that misdiagnosis occurs in clinical practice (e.g., underdiagnosis)? Should patients who are pre-symptomatic be treated considering the mechanism of action of the drug under review?
Response: Patients are identified by biomarker testing (MMR or MSI testing) which is performed on all uterine cancers in Canada. This would identify eligible patients for this therapy once they progressed on platinum-based therapy. Which patients would be least suitable for treatment with the drug under review?
Which patients would be least suitable for treatment with the drug under review?
Response: Patients with pMMR endometrial cancer would not be as suitable for this monotherapy treatment.
Is it possible to identify those patients who are most likely to exhibit a response to treatment with the drug under review? If so, how would these patients be identified?
Response: Yes, current standard of care in Canadian centres includes universal testing of all endometrial carcinomas for mismatch repair deficiency (MMR). This is a highly targeted and small population with a known biomarker (with established, validated testing) that identifies those who are most likely to respond.
What outcomes are used to determine whether a patient is responding to treatment in clinical practice?
Are the outcomes used in clinical practice aligned with the outcomes typically used in clinical trials?
Response: Standard clinical monitoring of therapy including physical exam and symptom review and intermittent imaging by CT scan.
What would be considered a clinically meaningful response to treatment?
Examples: Reduction in the frequency or severity of symptoms (provide specifics regarding changes in frequency, severity, and so forth). Attainment of major motor milestones. Ability to perform activities of daily living. Improvement in symptoms. Stabilization (no deterioration) of symptoms. Consider the magnitude of the response to treatment. Is this likely to vary across physicians?
Response: Clinically meaningful response would be improvement of symptoms and physical findings related to the recurrent endometrial cancer (pain, bleeding, shortness of breath, etc), while maintaining or improving performance status and ability to perform activities of daily living. Evidence of disease regression on imaging studies would be used to support the clinical findings. In many cases, disease stabilization is also a very meaningful endpoint, especially if the patient had a good baseline performance status and few disease-related symptoms.
How often should treatment response be assessed?
Response: Initial monitoring every 3 weeks following treatment initiation to assess for treatment tolerance and assessment of toxicities (diarrhea, hypertension, hypothyroidism, fatigue, anemia). Imaging would generally be ordered every 8-12 weeks by local standards.
What factors should be considered when deciding to discontinue treatment?
Examples: Disease progression (specify, e.g., loss of lower limb mobility). Certain adverse events occur (specify type, frequency, and severity). Additional treatment becomes necessary (specify).
Response: Treatment should be discontinued if patients experience disease progression or serious toxicities that preclude re-challenge with the same agent.
What settings are appropriate for treatment with the drug under review?
Response: This therapy is suitable to be delivered in community, outpatient, and specialty clinics. Patients should be under the care of a treating physician with experience in the monitoring and evaluation of possible toxicities caused by immune checkpoint inhibitor therapy. This group of agents is now routinely used by most oncologists.
For non-oncology drugs, is a specialist required to diagnose, treat, and monitor patients who might receive the drug under review?
Response: N/A
Additional Information
Is there any additional information you feel is pertinent to this review?
Response: N/A
Conflict of Interest Declarations
To maintain the objectivity and credibility of the CADTH drug review programs, all participants in the drug review processes must disclose any real, potential, or perceived conflicts of interest. This conflict-of-interest declaration is required for participation. Declarations made do not negate or preclude the use of the clinician group input. CADTH may contact your group with further questions, as needed. Please see the Procedures for CADTH Drug Reimbursement Reviews (section 6.3) for further details.
Did you receive help from outside your clinician group to complete this submission? If yes, please detail the help and who provided it.
N/A
Did you receive help from outside your clinician group to collect or analyze any information used in this submission? If yes, please detail the help and who provided it.
N/A
List any companies or organizations that have provided your group with financial payment over the past two years AND who may have direct or indirect interest in the drug under review. Please note that this is required for each clinician that contributed to the input — please add more tables as needed (copy and paste). It is preferred for all declarations to be included in a single document.
Declaration for Clinician 1
Name: Jennifer Brown Broderick
Position: Gynecologic Oncologist
Date: 24-10-2021
Table 26
Conflict of Interest Declaration for the Society of Gynecologic Oncology of Canada Clinician 1.
Declaration for Clinician 2
Name: Alon Altman
Position: Gynecologic Oncologist
Date: 27-10-2021
Table 27
Conflict of Interest Declaration for the Society of Gynecologic Oncology of Canada Clinician 2.
Declaration for Clinician 3
Name: Michael Fung-Kee-Fung
Position: Gynecologic Oncologist
Date: 29-10-2021
Table 28
Conflict of Interest Declaration for the Society of Gynecologic Oncology of Canada Clinician 3.
Sunnybrook Health Sciences Centre, Division of Gynecologic Oncology
About Sunnybrook Health Sciences Centre, Division of Gynecologic Oncology
The division of gynecologic oncology at the Odette Cancer Centre, Sunnybrook Health Sciences includes five gynecologic oncologists and two medical oncologists. The goal of our division is to treat women with female genital tract cancer. All members of the division have academic appointments at the University of Toronto.
Information Gathering
The information for this submission was gathered by performing a literature search. This included searching previously published articles through PubMed and gathering information from abstract presentations. The information was gathered by the members of our division.
Further, our division has participated in multiple clinical trials in which the investigational drug was an immunotherapy, similar to Dostarlimab, and we therefore have experience in assessing the drug and the specific side effect profile of these agents.
Current Treatments
Focus on the Canadian context. Please include drug and non-drug treatments. Drugs without Health Canada approval for use in the management of the indication of interest may be relevant if they are routinely used in Canadian clinical practice. Are such treatments supported by clinical practice guidelines? Treatments available through special access programs are relevant. Do current treatments modify the underlying disease mechanism? Target symptoms?
Response: Treatment of patients with recurrent endometrial cancer who have previously received one line of platinum-based chemotherapy include: second line chemotherapy, radiation therapy or surgery. The preferred modality is based on extent and location of disease at time of recurrence. In most cases of localized pelvic recurrence in patients that have not previously received radiation treatment, pelvic radiation is the preferred modality. In most cases, when disease recurrence extends beyond the pelvis systemic therapy is the preferred option. Currently, the main options for systemic therapy include endocrine therapy (progesterone) for low grade endometrial cancer and chemotherapy for recurrent high- grade cancer. Multiple single agent chemotherapy regimens including; liposomal doxorubicin, topotecan, oxaliplatin, docetaxel and bevacizumab have been assessed in clinical trials with an overall response rate estimated at 7 to 20% and a duration of response less than six months. Due to the low response rates with a limited duration of response, new treatments are needed. The combination of Pembrolizumab and Lenvatinib has demonstrated promising results in patients with recurrent endometrial cancer. In a study including 108 patients an overall response rate of 38% was demonstrated; among subgroups the response rate was 63.6% in MSI-H tumours and 36.2% in MSI-S tumours. The median duration of response was 21.2 months. However, the side effect profile was significant with 66.9% of patients experiencing a grade 3 or 4 toxicity. It is currently not funded in Canada.
Treatment Goals
Examples: Prolong life, delay disease progression, improve lung function, prevent the need for organ transplant, prevent infection or transmission of disease, reduce loss of cognition, reduce the severity of symptoms, minimize adverse effects, improve health-related quality of life, increase the ability to maintain employment, maintain independence, reduce burden on caregivers.
Response: The goal of treatment in recurrent endometrial cancer is to prolong progression free survival while maintaining a good quality of life. This can be challenging to achieve when using standard chemotherapy regimens, especially when multi-regimen chemotherapy is used where significant toxicity is seen.
Treatment Gaps (Unmet Needs)
Considering the treatment goals in Section 4, please describe goals (needs) that are not being met by currently available treatments.
Examples: Not all patients respond to available treatments. Patients become refractory to current treatment options. No treatments are available to reverse the course of disease. No treatments are available to address key outcomes. Treatments are needed that are better tolerated. Treatment are needed to improve compliance. Formulations are needed to improve convenience.
Response: The current available treatments for recurrent endometrial cancer lack both efficacy and a durable response. Those that have had previous chemotherapy treatment have a low response rate of around 10- 15% and the duration of response is limited.
Which patients have the greatest unmet need for an intervention such as the drug under review?
Would these patients be considered a subpopulation or niche population? Describe characteristics of this patient population. Would the drug under review address the unmet need in this patient population?
Response: Endometrial cancer has been traditionally categorized using histologic subtype. Recently, molecular characterization has provided a better understanding of endometrial cancer from a prognosis perspective and potentially can better predict response to treatment. Hence, the option to treat patients with targeted therapy using molecular characterization is an exciting opportunity that has the potential to identify treatment options that will produce a high response rate.
Patients included in this submission are those with a MMR deficiency (dMMR). dMMR is found in approximately 35% of patients with endometrial cancer and therefore, although it represents a subgroup it includes a high percentage of patients.
Further, the patient population included patients with recurrent endometrial cancer that were previously treated with a platinum based doublet chemotherapy and that had ≤2 lines of chemotherapy.
Place In Therapy
How would the drug under review fit into the current treatment paradigm?
Is there a mechanism of action that would complement other available treatments, and would it be added to other treatments? Is the drug under review the first treatment approved that will address the underlying disease process rather than being a symptomatic management therapy? Would the drug under review be used as a first-line treatment, in combination with other treatments, or as a later (or last) line of treatment? Is the drug under review expected to cause a shift in the current treatment paradigm?
Response: The role of Dostarlimab would be for treatment of patients with recurrent endometrial cancer that are MMR deficient and have previously been treated with one line of platinum based chemotherapy. These patients currently have very limited options and therefore Dostarlimab offers an excellent treatment option with a limited and tolerable side effect profile making it a better option for our patients.
Please indicate whether or not it would be appropriate to recommend that patients try other treatments before initiating treatment with the drug under review. Please provide a rationale from your perspective.
Response: Of the 71 evaluable patients with dMMR endometrial cancer that were enrolled in the GARNET study the overall response rate was 42.3% with 12.7% of patients achieving a complete response. They not only demonstrated a high response rate but also a durable response with 93.3% of patients demonstrating a duration of response longer than six months. The average duration of response for second line chemotherapy is around 6 months. Dostarlimab was well tolerated with only 5.6% of patients discontinuing drug due to side effects. Hence, Dostarlimab provides an excellent second line option (after platinum-based chemotherapy) for patients with dMMR and should be considered the preferred treatment regimen. Further, given the poor response rates demonstrated in previous studies and few treatment options available for these patients we find this data compelling and hence, strongly agree that Dostarlimab should be made accessible to our patients.
How would this drug affect the sequencing of therapies for the target condition?
If appropriate for this condition, please indicate which treatments would be given after the therapy has failed and specify whether this is a significant departure from the sequence employed in current practice. Would there be opportunity to treat patients with this same drug in a subsequent line of therapy? If so, according to what parameters?
Response: Dostarlimab would replace the current standard of care for patients with recurrent endometrial cancer that are dMMR that have been treated with platinum-based chemotherapy. Patients that progress on Dostarlimab would then be eligible for second line chemotherapy.
Which patients would be best suited for treatment with the drug under review?
Which patients are most likely to respond to treatment with the drug under review? Which patients are most in need of an intervention? Would this differ based on any disease characteristics (e.g., presence or absence of certain symptoms, stage of disease)?
Response: Patients that are best suited for treatment with Dostarlimab are those with recurrent endometrial cancer that have received a prior line of platinum-based chemotherapy but no more than 2 prior lines of treatment and have a documented dMMR as assessed by IHC. These patients have a 42.3% chance of response and a 93.3% chance of a duration of response six months or longer.
How would patients best suited for treatment with the drug under review be identified?
Examples: Clinician examination or judgement, laboratory tests (specify), diagnostic tools (specify). Is the condition challenging to diagnose in routine clinical practice? Are there any issues related to diagnosis? (e.g., tests may not be widely available, tests may be available at a cost, uncertainty in testing, unclear whether a scale is accurate or the scale may be subjective, variability in expert opinion.). Is it likely that misdiagnosis occurs in clinical practice (e.g., underdiagnosis)? Should patients who are pre-symptomatic be treated considering the mechanism of action of the drug under review?
Response: Patients with recurrent endometrial cancer are treated by gynecologic oncologists or medical oncologists at cancer centres. These patients will be identified by their treating oncologist. Progression of disease will be determined by a combination of clinical symptoms and radiologic evidence on CT scan. A biopsy can be obtained in cases in which the diagnosis of recurrent endometrial cancer is uncertain.
Which patients would be least suitable for treatment with the drug under review?
Response: Patients with endometrial cancer that would be less suitable for treatment with the Dostarlimab include those that have not yet been treated with platinum based chemotherapy and patients without mismatch repair deficiency as seen on IHC. Further, patients with a poor performance status (ECOG 3 or above), inadequate organ function, those with a poor medical risk due to a serious uncontrolled medical disorder and those with a known immunodeficiency or currently on systemic steroids or other immunosuppressant medications.
Is it possible to identify those patients who are most likely to exhibit a response to treatment with the drug under review?
Response: In the GARNET study responses to Dostarlimab were seen in patients with type I and II endometrial cancer 40% and 47.6%, respectively), in those with 1 or 2 lines of previous chemotherapy and in 50% of those with a response to their most recent platinum based chemotherapy. Hence, all patients with dMMR recurrent endometrial cancer that have had at least 1 but no more than 2 lines of prior chemotherapy should be offered treatment.
What outcomes are used to determine whether a patient is responding to treatment in clinical practice? Are the outcomes used in clinical practice aligned with the outcomes typically used in clinical trials?
Response: The outcome that was used in the GARNET trial is response to treatment as per RECIST v1.1 criteria. This is an acceptable means of assessing response in clinical practice and should be used in this case.
What would be considered a clinically meaningful response to treatment?
Examples: Reduction in the frequency or severity of symptoms (provide specifics regarding changes in frequency, severity, and so forth). Attainment of major motor milestones. Ability to perform activities of daily living. Improvement in symptoms. Stabilization (no deterioration) of symptoms. Consider the magnitude of the response to treatment. Is this likely to vary across physicians?
Response: A clinically meaningful response in this patient population would include improvement in symptoms e.g. a reduction in abdominal or pelvic pain, a reduction in urinary or bowel symptoms, increased appetite and energy levels. A progression free survival of 6 months or more would be clinically meaningful in this patient population.
How often should treatment response be assessed?
Response: Response should be assessed on imaging (CT scan) every 12 weeks. Due to the nature of immunotherapies and the possibility of pseudoprogression, patients with progression of disease on the first CT scan after initiation of treatment, but no other symptoms, should continue treatment until further imaging demonstrates progression of disease.
What factors should be considered when deciding to discontinue treatment?
Examples: Disease progression (specify; e.g., loss of lower limb mobility). Certain adverse events occur (specify type, frequency, and severity). Additional treatment becomes necessary (specify)
Response: Dostarlimab should be discontinued if radiologic evidence of unequivocal progression of disease is demonstrated or if patients develop intolerable side effects. The main side effects that warrant consideration of holding or discontinuing Dostarlimab are: grade 3 anemia, pneumonitis, grade 3 colitis, grade 3 asthenia, grade 3 myalgia, pemphigoid or grade 3 increase in transaminases.
What settings are appropriate for treatment with the drug under review?
Response: Dostarlimab should be administered in a chemotherapy suite with the appropriate supervision of an oncologist familiar with immunotherapy side effects.
For non-oncology drugs, is a specialist required to diagnose, treat, and monitor patients who might receive the drug under review?
If so, which specialties would be relevant?
Response: N/A
Additional Information
Is there any additional information you feel is pertinent to this review?
Response: No
Conflict of Interest Declarations
To maintain the objectivity and credibility of the CADTH drug review programs, all participants in the drug review processes must disclose any real, potential, or perceived conflicts of interest. This conflict of interest declaration is required for participation. Declarations made do not negate or preclude the use of the clinician group input. CADTH may contact your group with further questions, as needed. Please see the Procedures for CADTH Drug Reimbursement Reviews (section 6.3) for further details.
Did you receive help from outside your clinician group to complete this submission? If yes, please detail the help and who provided it.
No outside help was provided.
Did you receive help from outside your clinician group to collect or analyze any information used in this submission? If yes, please detail the help and who provided it.
No help was provided in analyzing or interpreting the data.
List any companies or organizations that have provided your group with financial payment over the past two years AND who may have direct or indirect interest in the drug under review. Please note that this is required for each clinician that contributed to the input — please add more tables as needed (copy and paste). It is preferred for all declarations to be included in a single document.
Declaration for Clinician 1
Name: Danielle Vicus
Position: Staff Gynecologic Oncologist
Date: 26-10-2021
Table 29
Conflict of Interest Declaration for Sunnybrook Health Sciences Centre, Division of Gynecologic Oncology Clinician 1.
Declaration for Clinician 2
Name: Al Covens
Position: Department Head, Gynecologic Oncology
Date: 26-10-2021
Table 30
Conflict of Interest Declaration for Sunnybrook Health Sciences Centre, Division of Gynecologic Oncology Clinician 2.
Declaration for Clinician 3
Name: Lilian Gien
Position: Gynecologic Oncologist, Gynecology Site Group Lead Odette Cancer Centre
Date: 29/10/2021
Table 31
Conflict of Interest Declaration for Sunnybrook Health Sciences Centre, Division of Gynecologic Oncology Clinician 3.
Declaration for Clinician 4
Name: Raymond Osborne
Position: Staff Gynecologic Oncologist
Date: 29-10-2021
Table 32
Conflict of Interest Declaration for Sunnybrook Health Sciences Centre, Division of Gynecologic Oncology Clinician 4.
Declaration for Clinician 5
Name: Rachel Kupets
Position: Staff Gynecologic Oncologist
Date: 29-10-2021
Table 33
Conflict of Interest Declaration for Sunnybrook Health Sciences Centre, Division of Gynecologic Oncology Clinician 5.
- Stakeholder Input - Dostarlimab (Jemperli)Stakeholder Input - Dostarlimab (Jemperli)
Your browsing activity is empty.
Activity recording is turned off.
See more...