Clinical Description
The clinically significant phenotypes of alpha-thalassemia (α-thalassemia) are hemoglobin Bart hydrops fetalis (Hb Bart) syndrome and hemoglobin H (HbH) disease. The severity of the α-thalassemia syndromes depends on the extent of alpha globin (α-globin) chain defect (see Genotype-Phenotype Correlations).
Hb Bart syndrome is the most severe clinical condition related to α-thalassemia. Affected fetuses are either delivered stillborn at 30-40 weeks' gestation or die soon after birth.
The main clinical features are generalized edema and pleural and pericardial effusions as a result of congestive heart failure induced by severe anemia. Notably, red cells with Hb Bart have an extremely high oxygen affinity and are incapable of effective oxygen delivery. Extramedullary erythropoiesis, marked hepatosplenomegaly, and a massive placenta are common.
Retardation in brain growth, hydrocephalus, cardiovascular deformities, and urogenital defects have been reported.
A very small number of newborns survive following intrauterine transfusions and repeated frequent transfusions after birth.
Maternal complications during pregnancy commonly include preeclampsia, polyhydramnios or oligohydramnios, antepartum hemorrhage, and premature delivery.
HbH disease. The phenotype of HbH disease varies; however, clinical features are usually only diagnosed during routine hematologic analysis in an asymptomatic individual.
The majority of individuals have enlargement of the spleen and less commonly of the liver, mild jaundice, and sometimes mild-to-moderate thalassemia-like skeletal changes (e.g., hypertrophy of the maxilla, bossing of the skull, and prominence of the malar eminences) that affect the facial features. Leg ulcers are rare.
Individuals with HbH disease may develop gallstones and experience acute episodes of hemolysis in response to oxidant drugs and infections. Rarely, infection with parvovirus B19 can cause an aplastic crisis.
While the majority of individuals with HbH disease have minor disability, some are severely affected, requiring regular blood transfusions; in very rare cases hydrops fetalis is present [Lorey et al 2001, Chui et al 2003].
Significant iron overload is uncommon but has been reported in older individuals, usually resulting from repeated blood transfusions or increased iron absorption [Taher et al 2012].
Nomenclature
The α-thalassemia carrier states have been classified on the basis of the total globin protein produced from each of the two α-globin genes and by the number of globin genes that are missing or abnormal (see Table 4).
Table 4.
Carrier State Nomenclature
View in own window
Number of Deleted/
Inactivated α-Globin Genes | Nomenclature
Based on #
of Deleted/
Inactivated α-
Globin Genes | Haplotype
(i.e., cis or trans) 1 | Genotype Example | Nomenclature Based on Protein 2 | Carrier State Terminology |
|---|
| Symbol | Definition |
|---|
| 1 | α-thalassemia silent carrier 3 | NA | -α/αα 4 | α+ | Some α-globin protein is produced from one chromosome 16. | α-thalassemia silent carrier |
| 2 | α-thalassemia trait/carrier 3 |
Cis
| --/αα | α0 | Zero α-globin protein is produced from one chromosome 16. | α0 trait (α0-thalassemia) |
|
Trans
| -α/-α | α+ | Some α-globin protein is produced from each of two chromosomes 16. | α+-thalassemia trait |
- 1.
Cis: both α-globin genes on one chromosome 16 are deleted or inactivated; trans: one α-globin gene on one chromosome 16 is deleted or inactivated by a non-deletion variant.
- 2.
HBA2 encodes two to three times more globin than HBA1.
- 3.
- 4.
The most common genotypes are the -α3.7 and -α4.2 deletion alleles (see Table 6 and Table 10).
Genotype nomenclature. In the expression αα/αα, the first alpha in each pair (αα/αα) typically refers to HBA2 and the second alpha in each pair (αα/αα) to HBA1.
The terms "α-thalassemia 1" and "α-thalassemia 2" (referring to α-thalassemia silent carrier and α-thalassemia trait, respectively) are no longer in use [Weatherall et al 1988].
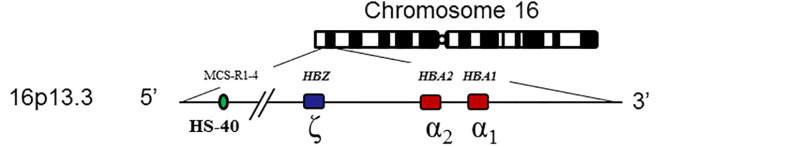
MCS-R2, a multispecies conserved sequence previously known as HS-40, is a cis-acting regulatory element about 40 kb upstream of HBZ that is required for α-globin expression [reviewed by Farashi & Harteveld 2018] (see ).
Prevalence
Since the early 1960s, prevalence of α-thalassemia has been determined in several populations using the percent of Hb Bart in cord blood. However, because not all newborns with α-thalassemia (mainly α-thalassemia silent carriers) have increased Hb Bart, the prevalence of α-thalassemia derived from this measure may be underestimated.
Data that are more precise have been obtained using molecular testing. For detailed references for the frequency of α-thalassemia in each population, see Piel & Weatherall [2014].
Africa
The highest allele frequency (0.30-0.40) of the -α3.7 allele has been observed in the equatorial belt including Nigeria, Ivory Coast, and Kenya.
Deletion of the two α-globin genes in cis (--/αα) has been reported very rarely in North Africa and in the African American population.
The Mediterranean
Alpha-thalassemia trait caused by -α3.7/-α3.7 is common, with the highest allele frequency reported in Sardinia (0.18) and the lowest in Spain.
Deletion of the two α-globin genes in cis (--/αα) is very rare (0.002); thus, Hb Bart hydrops fetalis is only rarely reported.
A remarkable aspect of α-thalassemia variants identified in the Mediterranean population is the heterogeneity of variants, particularly the non-deletion variants.
The Arabian Peninsula
Frequency of the -α3.7 allele (causing α-thalassemia trait) varies from 0.01 to 0.67, with the highest values being observed in Oman.
Deletion of the two α-globin genes in cis (--/αα) is extremely rare.
India
Alpha-thalassemia trait reaches very high allele frequency (0.35-0.92) in the Indian tribal population of Andra Pradesh; in other tribes, the frequency is much lower (0.03-0.12). Both the -α3.7 allele and the -α4.2 allele variably contribute to incidence of α-thalassemia trait.
Deletion of the two α-globin genes in cis (--/αα) is very rare.
Southeast Asia
Alpha0-thalassemia alleles (--SEA, --THAI, --FIL) and α+-thalassemia alleles (-α) are very common, causing a major public health burden.
Alpha-thalassemia caused by Hb Constant Spring alleles is also common.
The incidence of Hb Bart hydrops fetalis is expected to be in the range of 0.5-5:1,000 births and HbH disease the range of 4-20:1,000 births.
Oceania
The distribution of α-thalassemia, extensively studied by DNA-based methods, follows a pattern consistent with the degree of malaria endemicity. The prevalence of α-thalassemia is low in the highlands and high in the coastal areas and the lowlands where malaria is hyperendemic.
Some α-thalassemias have unusual mutation mechanisms; for example, some affected individuals on the island of Vanuatu who have normal α-globin genes without deletions or variants have a variant in a regulatory element that creates a GATA-1 site and activates a cryptic promoter [De Gobbi et al 2006].
Deletion of the two α-globin genes in cis (--/αα) is very rare.