NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Wider application and technical improvement of abdominal imaging procedures in recent years, has led to the discovery of unsuspected adrenal tumors in an increasing frequency. These incidentally detected lesions, also called adrenal incidentalomas, have become a common clinical problem and need to be investigated for evidence of hormonal hypersecretion and/or malignancy. In this chapter, current information on the prevalence, etiology, radiological features, and appropriate biochemical evaluation are presented as a narrative review of the available literature. Despite the flurry of data accumulated, controversies are still present regarding the accuracy of diagnostic tests and cut-offs utilized to establish hormonal hypersecretion, potential long-term sequelae, indications for surgical treatment as well as duration and intensity of conservative management and follow-up. Recently, clinical guidelines proposing diagnostic and therapeutic algorithms have been published to aid in clinical practice, however an individualized approach through a multidisciplinary team of experts is recommended. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
Abdominal computed tomography (CT), since its introduction in the late 1970’s, has proven to be an excellent tool for identifying pathology in patients with suspected adrenal disease. It was also predicted that the ability of CT to image both adrenal glands could lead to the occasional discovery of asymptomatic adrenal disease (1). Nowadays, further technological advances and broader availability of CT and other imaging modalities such as Ultrasonography (US), Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET) have made the detection of unexpected lesions in adrenal and other endocrine glands a common finding (2). Although incidental detection of adrenal disease may lead to earlier diagnosis and possibly improved outcome in certain cases, it is now recognized that diagnostic evaluation and follow-up of all incidentally discovered adrenal masses, or so-called “adrenal incidentalomas”, may put a significant burden on patient’s anxiety and health and produce increasing financial consequences for the health system (3). It is therefore important to develop cost-effective strategies to diagnose and manage patients with adrenal incidentalomas.
DEFINITION
According to the NIH State-of-the-Science Statement (4), adrenal incidentalomas (AIs) are defined as clinically inapparent adrenal masses discovered serendipitously during diagnostic testing or treatment for conditions not related to the adrenals, such as abdominal or back pain or for exclusion of pulmonary embolism or other lung disease. Although an arbitrary cut-off of 1 cm or more has been employed to define an adrenal lesion as AI (5,6), this cut-off might be challenged following the higher resolution that modern imaging modalities offer, mainly MRI and CT. Nonetheless, in all published guidelines this cut-off is accepted as the minimum size above which additional diagnostic work-up should be performed, unless clinical signs and symptoms suggestive of adrenal hormone excess are present. Patients harboring an AI, by definition, should not have any history, signs, or symptoms of adrenal disease prior to the imaging procedure that led to its discovery. This strict definition excludes cases in which adrenal-specific signs and symptoms are “missed” during history taking or physical examination, or in which a hereditary syndrome associated with an increased likelihood to develop adrenal tumors is suspected (6). Similarly, adrenal masses discovered on imaging for tumor staging or follow-up in extra-adrenal malignancies fall outside the definition of an AI (7). This is because adrenal metastases are a common finding in this setting, with a prevalence ranging from 3 to 40% in autopsy and from 6 to 20% in radiological series (8). A recent population-based cohort study reported a 22-fold higher likelihood of an AI being a metastatic lesion when discovered during cancer staging, reaching a prevalence of 7.5% (9). In another single-center cohort study including 475 patients with colorectal cancer, the incidence of AIs was 10.5% (10).
EPIDEMIOLOGY
The precise prevalence and incidence of AIs cannot be easily defined since data from population-based studies are scarce. Most previous data were extrapolated from autopsy or radiological studies that are inherently biased due to their retrospective nature, insufficient clinical information, different referral patterns and patient selection criteria.
In autopsy studies, the reported prevalence of AIs was found to be around 2.3%, ranging from 1 to 8.7% (11–23), without any significant gender difference. The prevalence of AIs increases with age, being 0.2% in young subjects compared to 6.9% in subjects older than 70 years of age (24), and is higher in white, obese, diabetic, and hypertensive patients (8). The variability of the reported prevalence in different series could also be attributed to the size cut-off used for the definition of AI as in some post-mortem series, small nodules (<1 cm) were detected in more than half of the patients examined (23).
In radiological studies, the prevalence of AIs differs depending on the imaging modality used and should be interpreted carefully due to referral and under-reporting bias. Transabdominal US during a routine health examination identified AIs in 0.1% of those screened (25), while studies using CT reported a mean prevalence of 0.64% ranging from 0.35 to 1.9% in a total of 82,483 scans published in the literature between 1982 and 1994 (21,26–30). However, two more recent studies utilizing high-resolution CT scanning technology, have reported prevalence rates of 4.4% and 5% respectively, which are more consistent with those observed in autopsy studies (31,32). This increase in detection frequency paralleled by the technological advances in medical imaging quality, can explain why AIs are considered a “disease of modern technology”. Age has also been found to affect AI radiological detection rates, as these lesions are found in 0.2% of individuals younger than 30 years, in 3% at the age of 50 years and up to 10% in individuals above 70 years of age (24,31,33). However, a recent publication from China including 25,356 healthy individuals (aged 18-78) who underwent abdominal CT imaging as part of a funded health check, reported an AI detection rate of 1.4%, increasing with age, from 0.2% in the youngest group (18-25 years) to 3.2% in those older than 65 years (34). The prevalence of AIs is very low in childhood and adolescence accounting for 0.3-0.4% of all tumors (35). Adrenal incidentalomas appear to be slightly more frequent in women in radiological series, in discordance with autopsy studies, probably because women undergo abdominal imaging more frequently than men (33). Bilateral AIs are found in 10-15% of cases (36), while distribution between the two adrenals appears to be similar in both post-mortem and CT studies (8,33).
DIFFERENTIAL DIAGNOSIS
Adrenal Incidentalomas are not a single pathological entity, but rather comprise a spectrum of different pathologies that share the same path of discovery and include both benign and malignant lesions arising from the adrenal cortex, the medulla, or being of extra-adrenal origin (Table 1).
Table 1.
The Spectrum of Lesions Presenting as AIs, Modified from (37)
| Adrenal Cortex lesions |
| Adenoma (non-functioning) |
| Adenoma (functioning) |
| Cortisol-secreting (MACS) |
| Aldosterone-secreting |
| Nodular hyperplasia (primary bilateral macronodular adrenal hyperplasia)* |
| Adrenocortical Carcinoma (secreting or non-secreting) |
| Adrenal Medulla lesions |
| Pheochromocytoma (benign or malignant)* |
| Ganglioneuroma |
| Neuroblastoma, ganglioneuroblastoma |
| Other adrenal lesions |
| Myelolipoma, lipoma |
| Hemangioma, angiosarcoma |
| Cyst |
| Hamartoma, teratoma |
| Metastases* (lung, breast, kidney, melanoma, lymphoma) |
| Infiltration* |
| Amyloidosis |
| Sarcoidosis |
| Lymphoma |
| Infections* |
| Abscess |
| Fungal/parasitic (histoplasmosis, coccidiomycosis, tuberculosis) |
| Cytomegalovirus |
| Adrenal hemorrhage or hematomas* |
| Adrenal pseudotumors |
| Congenital Adrenal Hyperplasia (CAH)* |
- *
Should be considered when bilateral adrenal lesions are detected.
In general, the vast majority (80-90%) of AIs are benign adrenal adenomas, as shown by accumulated follow-up data from their natural history, even in the absence of pathological confirmation, since adrenal adenomas are rarely excised (5). However, a number of these lesions may be malignant and/or exhibit autonomous hormonal secretion that is not clinically detected due to subtle secretory pattern or periodical secretion. Therefore, the task a physician faces when dealing with an AI is mainly to exclude malignant and functioning tumors.
Mild autonomous cortisol secretion (MACS) is the most frequent endocrine dysfunction detected in patients with AIs, with a prevalence ranging from 5 to 30%, depending on the study design, work-up protocols, and mainly diagnostic criteria used (5). This condition exclusively identified in the setting of AIs, also termed subclinical Cushing’s syndrome or subclinical hypercortisolism, is characterized by the absence of the typical clinical phenotype of hypercortisolism and by the presence of subtle alterations of the hypothalamic-pituitary-adrenal (HPA) axis. These tumors do not secrete cortisol under the physiological control of corticotropin (ACTH), but rather autonomously and in some cases under the control of one or more aberrant hormone receptors (38,39).
Pheochromocytomas (PCCs), albeit rare in the general population, are discovered in approximately 5% of patients with AIs (40), while more than 30% of PCCs are diagnosed as AIs (41). Clinical manifestations are highly variable, and the classic clinical triad (headache, palpitations and diaphoresis) is not present in most patients. In addition, several patients harbor ‘‘silent pheochromocytomas’’, being totally asymptomatic or having intermittent and subtle symptoms. In a large multicentric study, approximately half of the patients with PCCs presenting as AIs were normotensive, whereas the remaining had mild to moderate hypertension (33).
Primary aldosteronism (PA) has a median prevalence of 2% (range 1.1-10%) among patients with AIs (42). After excluding cases with severe hypertension and hypokalemia a retrospective study found that 16 out of 1004 subjects with AIs (1.5%) had PA (33). This figure is relatively low when compared to the prevalence of PA in unselected hypertensive populations which ranges from 4.6 to 16.6% (43) and may be related to the different investigational protocols and cut-offs indicative of autonomous aldosterone secretion used. The absence of hypokalemia does not exclude this condition, but absence of hypertension makes PA unlikely, although normotensive patients with PA have occasionally been reported (44). A recent study using a new diagnostic approach, considering the stimulatory effect that adrenocorticotropin (ACTH) could exert on aldosterone secretion, revealed a 12% prevalence of PA in normotensive and normokalemic patients with AIs (45).
Over secretion of adrenal androgens is usually accompanied with clinical signs or symptoms of virilization in women and feminization in men (46), thus falling out of the strict definition of AI’s requiring absence of adrenal-related manifestations. Presence of elevated adrenal androgens should alert the physicians for the possibility of an adrenocortical carcinoma, although benign androgen-secreting tumors have rarely been reported (47).
Combining studies that used a broad definition of incidentaloma without clearly stated inclusion criteria and those that reported descriptions of individual cases, Mansmann et al found 41% of AIs to be adenomas, 19% metastases, 10% ACCs, 9% myelolipomas, and 8% PCCs, with other benign lesions, such as adrenal cysts, ganglioneuromas, hematomas, and infectious or infiltrative lesions representing rare pathologies (48). However, the relative prevalence of any pathology depends on the inclusion criteria used and is highly influenced by referral bias. Surgical series and data from referral centers tend to overestimate the prevalence of large, malignant and functioning tumors, because such cases are mainly referred for surgery or expert evaluation. Similarly, metastatic lesions are much more common when patients with known extra-adrenal cancer are included in the study population. The probability of an incidentally discovered adrenal lesion in a patient without a history of cancer to be metastatic is as low as 0.4% (29). Studies applying more strict inclusion criteria may identify a greater number of small and biochemically silent tumors. In a comprehensive review, Cawood et al. (3) concluded that the prevalence of malignant and functioning lesions among AIs is likely lower when strict inclusion and exclusion criteria for the study populations are used. By analyzing 9 studies that more accurately simulated the clinical scenario of a patient referred for assessment of an AI, they reported a mean prevalence of 88.1% (range 86.4-93%) for non-functioning benign adrenal adenomas (NFAIs), 6% (range 4-8.3%) for MACS, 1.2% for aldosterinomas, 1.4% (range 0.8-3%) for ACCs, 0.2% (range 0-1.4%) for metastases and 3% (range 1.8-4.3%) for PCCs. These low rates for clinically significant tumors compared to those reported by previous studies (6,8,48), highlight the limitations of epidemiological data and raise significant questions concerning the appropriate diagnostic and follow-up protocols. Notably, it has recently been suggested that a significant number of patients with small AIs do not undergo the recommended evaluation (9), adding further confusion in defining the relative prevalence of each pathology, through under-reporting bias.
In the case of bilateral AIs, a broader spectrum of diagnoses needs to be considered (Table 1), particularly in a relevant clinical setting, including metastatic or infiltrative diseases of the adrenals, hemorrhage, congenital adrenal hyperplasia (CAH), bilateral cortical adenomas or PCCs, and primary bilateral macronodular adrenal hyperplasia (PBMAH) (49). Occasionally, adrenal tumors of different nature may simultaneously be present in the same patient or in the same adrenal gland (50–53). Adrenal pseudotumor is a term used to describe radiological images of masses that seem to be of adrenal origin, but arise from adjacent structures, such as the kidney, spleen, pancreas, vessels and lymph nodes or are results of technical artifacts.
PATHOGENESIS
The pathogenesis of AIs is largely unknown. Early observations in autopsy studies which revealed that AIs are more frequent in older patients, led to the notion that these tumors are a manifestation of the ageing adrenal and could represent focal hyperplasia in response to ischemic injury, a concept that was supported by histopathological findings of capsular arteriopathy (54). Clonal analysis of adrenal tumors later revealed that the vast majority are of monoclonal origin and only a few arise from polyclonal focal nodular hyperplasia under the putative effect of local or extra-adrenal growth factors (55,56). In this sense, it has been postulated that hyperinsulinemia associated with the insulin resistance in individuals with the metabolic syndrome, which frequently coexists in patients harboring AIs, could contribute to the development of these tumors, through the mitogenic action of insulin on the adrenal cortex (57,58). However, the opposite causal relationship, that subtle autonomous cortisol production from AIs results in insulin resistance, has also been proposed (59). It is plausible that both pathways can be true in a reciprocal triad. Another interesting hypothesis involves alterations in the glucocorticoid feedback sensitivity of the HPA axis acting as a drive for adrenal cell proliferation especially in cases with bilateral involvement. In a recent study, unexpected ACTH and cortisol responses to the combined dexamethasone-CRH (corticotropin-releasing hormone) test were found, in about half of the patients with bilateral AIs, when compared to control and unilateral adenoma cases (60). Such a dysregulated ACTH secretion during lifetime may lead to subtle but chronic trophic stimulation of the adrenals by repeatedly inappropriately higher ACTH levels, particularly in response to stress, favoring nodular adrenal hyperplasia.
Although several genetic syndromes are known to be associated with adrenal tumors, germline or somatic genetic alterations are identified only in subgroups of sporadic tumors that are mainly functioning (61–63). Elucidation of specific signaling pathways involved in these familial syndromes has led to the identification of several mutations in genes not previously described in ACCs, cortisol- and aldosterone-secreting adenomas as well as PCCs, creating new insights in adrenal tumorigenesis (Figure 1). However, the genetics of benign NFAIs that account for the majority of AIs are poorly understood.

Figure 1.
Genes Involved in the Development of Adrenocortical Tumors IN Sporadic or Familial Cases. MEN: Multiple Endocrine Neoplasia; CTNNB1: Catenin Beta-1 gene; CYP21A2: 21-Hydroxylase gene; CAH: Congenital Adrenal Hyperplasia; APC: Adenomatous polyposis coli; FAP: Familial adenomatous polyposis; KCNJ5: gene encoding potassium channel, inwardly rectifying subfamily J, member 5; ATP1A1: gene encoding sodium/potassium-transporting ATPase subunit alpha 1; ATP2B3: plasma membrane calcium-transporting ATPase 3; CACNA1D: gene encoding calcium channel, voltage-dependent, L type, alpha 1D subunit; ARMCS: Armadillo repeat containing 5; ZNRF3: gene encoding Zinc and Ring Finger3; IGF-2: Insulin-like growth factor 2; TP53: tumor protein p53; CDKN2A: cyclin-dependent kinase inhibitor 2A; RB1: retinoblastoma protein; DAXX: death-associated protein 6; GNAS: gene encoding G-protein alpha subunit: PDE11A: phosphodiesterase 11A; PDE8B: phosphodiesterase 8B; PRKACA: gene encoding catalytic subunit alpha of protein kinase A; SDH-A-B-C-D: gene encoding succinate dehydrogenase complex subunit A, B, C, and D; SDHAF2: succinate dehydrogenase complex assembly factor 2; VHL: von-Hippel-Landau; RET: rearranged during transfection proto-oncogene; MAX: myc-associated factor X; TMEM127: gene encoding transmembrane protein 127.
DIAGNOSTIC APPROACH
Although the prevalence of potentially life-threatening disorders associated with AIs is relatively low, the question of whether a lesion is malignant (mainly an ACC) or functioning needs to be addressed in patients with an incidentally discovered adrenal mass. A careful clinical examination and a detailed medical history, evaluation of the imaging characteristics of the adrenal tumor(s), and biochemical evaluation to exclude hormonal excess can help clinicians identify the few cases that pose a significant risk and intervene accordingly.
CLINICAL EVALUATION
Per definition, patients with AIs should have no signs or symptoms implying adrenal dysfunction before the radiological detection of the adrenal tumor(s). In everyday clinical practice though, physicians who are not familiar with endocrine diseases may overlook mild signs of hormone excess and pursue evaluation of adrenal function following the incidental discovery of an adrenal mass. In this setting, such cases should not be designated as AIs and highlight the need for detailed and careful clinical history and examination (64).
IMAGING EVALUATION
Distinguishing malignant from benign AI lesions should be the priority at the time of their initial detection, and determination of their imaging phenotype is currently considered the most reliable and non-invasive approach to aid in this distinction. Traditionally the size of the lesion reported by CT or MRI has been considered as indicative of malignancy as most ACCs are large or significantly larger than adenomas at the time of diagnosis (33). In a meta-analysis, ACCs represented 2% of all tumors ≤4 cm in diameter, but the risk of malignancy increased significantly with tumor size greater than 4 cm, being 6% in tumors with size 4.1-6 cm and 25% in tumors >6 cm (65). However, size alone has low specificity in distinguishing benign from malignant lesions, since ACCs can also be relatively small during early stages of development and exhibit subsequent progressive growth (5). An analysis of 4 recent studies investigating the 4cm size cut-off to distinguish benign from malignant lesions reported sensitivities ranging from 23% to 90% while the pooled sensitivity was 77% (95% CI 45%-93%) and the pooled specificity was 90% (95% CI 78%-96%) (66). Other than size, findings suggestive of malignancy include irregular shape and borders, tumor heterogeneity with central necrosis or hemorrhage, and invasion into surrounding structures. Benign adenomas are usually small (<4 cm), homogenous, with well-defined margins. Slow growth rate or stable size of an adrenal mass have also been proposed as indicators of benign nature (4). However, studies on the natural history of AIs suggest that up to 25% of benign adenomas can display increase in size by almost 1 cm, while adrenal metastases with no change in CT appearance over a period of 36 months have been described, not allowing for the introduction of a safe cut-off of absolute growth or growth rate to distinguish benign from malignant lesions (67).
Computed Tomography (CT)
CT has a high spatial and contrast resolution, which allows assessment of tissue density by measuring X-ray absorption compared to water (attenuation, expressed in Hounsfield Units - HU). Water and air are conventionally allocated an attenuation value of 0 HU and -1000 HU respectively, while fat is usually characterized by a HU value between -40 and -100. Because there is an inverse linear relation between the fat content of a lesion and attenuation, lipid-rich adenomas express lower HU in unenhanced (without contrast medium) CT images compared to malignant lesions, which are usually lipid-poor (68). A value of ≤10 HU in unenhanced CT images is the most widely used and accepted attenuation threshold for the diagnosis of a lipid-rich, benign adrenal adenoma (69,70). In several studies a density of ≤10 HU was found to be superior to size in differentiating benign from malignant masses, displaying a sensitivity of 96-100% and a specificity of 50-100% (71). Data from 6 studies (9,72–76) on the diagnostic accuracy of unenhanced attenuation values, reported that a CT density >10 HU has a very high sensitivity for detection of adrenal malignancy (100% in all 6 studies), while the pooled specificity was clearly lower (56%-59%). This means that adrenal masses with a density of ≤10 HU are virtually never malignant, however a large number of benign lesions had HU > 10. Increasing the cutoff to HU > 20, provided a pooled sensitivity of 94%-98% and a higher specificity (75%-78%), leaving a fairly significant number of malignant tumors lying between 10 and 20 HU. In this context, the risk of malignancy in a homogeneous 5 cm adrenal mass with a CT attenuation value of 10 HU is close to 0% (49). On the other hand, up to 30-40% of benign adenomas are considered lipid-poor and have an attenuation value of >10 HU on non-contrast CT, which is considered indeterminate since it overlaps with those found in malignant lesions and PCCs. Hence, unenhanced CT attenuation is a useful screening tool to identify a lesion as benign and exclude malignancy but is less reliable in diagnosing a malignant mass with certainty. When considering patients with a history of extra-adrenal malignancy though, several studies evaluating the >10 HU cut-off as indicative of malignancy showed high sensitivity (93%) for the detection of malignancy but variable specificity, meaning that 7% of adrenal metastases were found to have a tumor density of ≤10 HU (70). Attenuation values in non-contrast CT can also reliably identify typical myelolipomas that have a density lower than -40 HU (49).
For those indeterminate adrenal lesions (>10HU) intravenous contrast administration reveals their hemodynamic and perfusion properties that can be utilized to distinguish benign from malignant lesions. The attenuation on delayed images (10-15 min post contrast administration) decreases more quickly in adenomas because they exhibit rapid uptake and clearance compared to malignant lesions that usually enhance rapidly but demonstrate a slower washout of contrast medium (77). There are two methods of estimating contrast medium washout: absolute percentage washout (APW) and relative percentage washout (RPW) and can be calculated from values of pre-contrast (PA), enhanced (EA, 60-70 seconds after contrast medium administration) and delayed (DA, 10-15 mins after contrast medium administration) attenuation values according to the formulas below:
APW=100 x (EA-DA) / (EA-PA)
RPW=100 x (EA-DA) / EA
Initial studies suggested that lipid-poor adenomas demonstrate rapid washout with APW >60% (sensitivity of 86-100%, specificity 83-92%) and a RPW >40% (sensitivity of 82-97%, specificity 92-100%) (78). Metastases usually demonstrate slower washout on delayed images (APW<60%, RPW<40%) than adenomas and ACCs typically have a RPW of <40% (79). It is important to note that the above values of sensitivity and specificity were produced in studies with limitations and high risk of bias due to the lack of definitive pathological diagnosis, different timing in acquiring post-contrast images, and the use of broad inclusion criteria, including not only AIs but also clinically overt adrenal masses. Recent data have suggested that these percentage washout cutoffs have suboptimal performance for characterizing benign lesions, since 22% (using APW) and 8% (using RPW) of malignant tumors are not correctly identified (70,75,80). To detect all malignant tumors, the RPW cutoff should be increased to 58%, leading to a specificity of only 15% (75).
Furthermore, contrast-enhanced washout CT studies may not suffice for characterization of lesions such as PCCs, cysts, and myelolipomas; in these cases, further biochemical, anatomical and/or functional imaging may be required. Findings consistent, but not diagnostic, of PCC on CT include high attenuation values, prominent vascularity, and delayed washout of contrast medium (79). Another recent study (81), showed that only a minority (21%) of cortisol-secreting adenomas has the typical unenhanced attenuation value of <10 HU, because cortisol secretion is associated with decreased intra-cytoplasmic lipid droplets containing cholesterol esters which are necessary for cortisol synthesis. Nevertheless, among the adenomas with high pre-contrast density (>10 HU), washout analysis after contrast administration was consistent with the benign nature of the tumor in 60% of the cases.
Another crucial key point in clinical practice is that most abdominal and chest CT scans leading to the unexpected discovery of an adrenal mass are obtained with the use of intravenous contrast that may not fulfill current technical recommendations for an optimal CT study of the adrenal glands, such as analysis on contiguous 3-5 mm-thick CT slices, preferentially on multiple sections using multidetector (MDCT) row protocols (82). In such cases, it may be worthwhile to obtain a new CT scan, specifically aimed for the study of the adrenal glands, including washout protocols in order to avoid the radiation exposure of a subsequent third CT scan in case of indeterminate unenhanced attenuation values.
Finally, the importance of thorough and standardized reporting by radiologists (including common terminology, nodule size, and HU) needs to be highlighted, in order to improve the percentage of patients with AIs that receive appropriate diagnostic testing and follow-up. This is a recently raised issue based on evidence that suggests that most of AIs are not adequately investigated according to international guidelines due to inconsistent use of terms and lack of specific details and recommendations in radiology reports (83–85).
Typical CT images of adrenal pathologies is shown in Figure 2.

Figure 2.
CT images of adrenal pathologies presenting as adrenal incidentalomas. a,b,c: A patient with a benign (lipid-rich) adrenal adenoma with unenhanced attenuation value - 3 HU (a), early attenuation (60 seconds after i.v. contrast medium administration) 35 HU (b) and delayed attenuation (10 min post-contrast administration) 18 HU. ARW = 45% and RPW=49%. Absolute washout (APW) less than 60% is indeterminate. However, the low pre-contrast attenuation is suggestive of an adenoma. Relative washout (RPW) of 40% or higher is consistent with an adenoma; d,e,f: Biochemically and histologically proven pheochromocytoma with unenhanced attenuation of 49 HU (d), early attenuation 90 HU (e) and delayed attenuation 64 HU. ARW = 63% and RPW=29%. Absolute washout >60% is suggestive of an adenoma, however relative washout less than 40% and unenhanced attenuation >10 HU are indeterminate; g,h: A patient with a primary adrenocortical carcinoma characterized by heterogeneity an unenhanced attenuation value >10 HU (g) and inhomogeneous contrast medium uptake due to central areas of necrosis; i: Typical myelolipoma.
Magnetic Resonance Imaging (MRI)
Adrenal imaging with MRI can also aid in the differential diagnosis between benign and malignant adrenal pathology. Benign adrenal adenomas appear hypotense or isotense compared to the liver on T1-weighted images and have low signal intensity on T2-weighted images. The majority of PCCs show high signal intensity on T2-weighted imaging (“light bulb sign”) which is a non-specific finding; however, a wide range of imaging features of PCCs mimicking both benign and malignant adrenal lesions have also been described (79). Primary ACCs are characterized by intermediate to high signal intensity on T1- and T2-weighted images and heterogeneity (mainly on T2- sequence due to hemorrhage and/or necrosis) as well as avid enhancement with delayed washout. However, these features are not specific and display significant overlap between benign and malignant lesions. The MRI technique of chemical-shift imaging (CSI) exploits the different resonance frequencies of protons of water and triglyceride molecules oscillating in- or out-of-phase to each other under the effect of specific magnetic field sequences, to identify high lipid content in adrenal lesions (86). Adrenal adenomas with a high content of intracellular lipids usually lose signal intensity in out-of-phase images compared to in-phase images, whereas lipid-poor adrenal adenomas, malignant lesions, and PCCs remain unchanged. Signal intensity loss can be assessed qualitatively by simple visual comparison or by quantitative analysis using the adrenal-to-spleen signal ratio and can identify adenomas with a sensitivity of 84-100% and a specificity of 92-100% (87). It must be noted however, that ACC and clear renal cell cancer metastases may sometimes also show signal loss (88).
The evidence regarding the diagnostic accuracy of MRI is generally considered poor for several reasons, such as: low number and quality of studies, lack of standardized quantitative assessment, subjective interpretation of qualitative loss in signal intensity, and paucity of recent high-quality research. Additionally, there are no good quality studies comparing the diagnostic performance of MRI and CT in AIs. Hence, based on the higher strength of evidence, CT is considered the primary radiological procedure for evaluating AIs, being also more easily available and cost-effective. MRI should be reserved for cases in which CT is less desirable (as in pregnant women and in children) (66,89).

Figure 3.
MRI images of different adrenal lesions presenting as incidentalomas, using the chemical shift imaging (CSI) technique. The loss of signal in out of phase images is typical in benign lipid-rich adenomas (a, b) in contrast with pheochromocytomas (c, d) and adrenocortical carcinomas (e, f) which do not display any signal loss.
Scintigraphy
In recent years, positron emission tomography (PET) using 18-fluoro-deoxyglucose (18F-FDG) has emerged as an effective tool in identifying malignant adrenal lesions. By utilizing the increased glucose uptake properties of cancer cells, 18F-FDG-PET combined with a CT scan (18F-FDG-PET/CT) achieves a sensitivity and specificity in identifying malignancy of 93-100% and 80-100% respectively (90,91). Both quantitative analysis of FDG uptake using maximum standardized uptake values (SUVmax) and qualitative assessment using a mass/liver SUV ratio have been used as a criterion, with the latter displaying better performance (92). A SUV ratio <1.45–1.6 between the adrenal and the liver is highly predictive of a benign lesion (93). Caveats in utilizing 18F-FDG-PET/CT include cost and availability, risk of false negative results in the case of necrotic or hemorrhagic malignant lesions, size <1cm, extra-adrenal malignancies with low uptake (such as metastases from renal cell cancer or low-grade lymphoma), and false positive results in cases of sarcoidosis, tuberculosis, and other inflammatory or infiltrative lesions and some adrenal adenomas and PCCs that show moderate FDG uptake (94). Because of its excellent negative predictive value, 18F-FDG-PET may help in avoiding unnecessary surgery in patients with non-secreting tumors with equivocal features in CT demonstrating low FDG uptake. Moreover, 18F-FDG-PET/CT may favor surgical removal of tumors with elevated uptake and no biochemical evidence of a PCC (90). Newer PET tracers such as 18F-fluorodihydroxyphenylalanine (F-DOPA) and 18F-fluorodopamine (FDA) for detection of PCC have also been developed but their availability is limited (95).
Conventional adrenal scintigraphy using radiolabeled cholesterol molecules such as 131I-6-b-iodomethyl-norcholesterol (NP-59) and 75Se-selenomethyl-19-norcholesterol has been used in the past to discriminate benign from malignant lesions. These tracers enter adrenal hormone synthetic pathways and act as precursor-like compounds, providing information regarding the function of target tissue. Typically, benign hypersecreting tumors, and non-secreting adenomas, show tracer uptake, whereas primary and secondary adrenal malignancies, space-occupying or infiltrative etiologies of AIs appear as ‘cold’ masses, providing an overall sensitivity of 71-100% and a specificity of 50-100% (96). However, some benign adrenal tumors such as myelolipomas and some functioning ACCs, may also be visualized with these modalities. Several additional limitations of adrenal scintigraphy such as insufficient spatial resolution, lack of widespread expertise, limited availability of the tracer, being a time-consuming procedure (which requires serial scanning over 5-7 days), and high radiation doses received by the patient, have limited its value in routine clinical practice, especially when conventional imaging can provide more reliable information. Recently, 123I-iodometomidate has been introduced as a tracer because it binds specifically to adrenocortical enzymes, but its application is hampered by its limited availability and heterogeneous uptake by ACCs (97). Scintigraphy with 123I-meta-iodo-benzyl-guanidine (MIBG) is the preferred method for identifying PCCs when clinical, biochemical, and imaging features are not conclusive, or when multiple or malignant lesions need to be excluded (40).
Table 2 summarizes the imaging properties of different underlying AI pathologies that can be helpful for the differential diagnosis.
Table 2.
Image Findings Differentiating Common Adrenal Pathologies in AIs
| FINDING | Benign adenoma | ACC | Pheochromocytoma | Metastases |
|---|---|---|---|---|
| Size | Usually <4cm | Usually >4cm | Variable | Variable |
| Growth rate | Stable or <0.8cm/year | Significant growth (>1cm/year) | Slow growth | Significant growth (>1cm/year) |
| Shape & margins | Round or oval with well-defined margins | Irregular shape and margins. Invasion to surrounding tissues | Variable | Variable |
| Composition | Homogenous | Heterogeneous (hemorrhage, necrosis) | Heterogeneous (necrosis) | Heterogeneous (hemorrhage, necrosis) |
| CT Unenhanced attenuation | ≤10 HU (or >10 HU for lipid-poor adenomas) | >10 HU | >10 HU | >10 HU |
| CT Percent Washout (PW) | APW >60% RPW>40% | APW<60%, RPW<40% | APW<60% RPW<40% | APW<60%, RPW<40% |
|
MRI – CSI
(out-of phase) | Signal loss (except in lipid-poor adenomas) | No change in signal intensity | No change in signal intensity | No change in signal intensity |
| FDG uptake (PET) | Low (some can have low to moderate uptake) | High | Low (malignant pheochromocytomas show high uptake) | High |
| NP-59 uptake | Present | Absent (except in some secreting tumors) | Absent | Absent |
ACC: Adrenocortical carcinoma; HU: Hounsfield Units; APW: Absolute PW; RPW: Relative PW; CSI: Chemical-shift Imaging; FDG: fluoro-deoxyglucose; NP-59: 131I-6-b-iodomethyl-norcholesterol.
HORMONAL EVALUATION
Patients with AIs should be screened at presentation for evidence of excess catecholamine or cortisol secretion and, if hypertensive and/or hypokalemic, for aldosterone excess. As already discussed, the definition of AI per se implies the absence of clinical symptoms/signs related to these entities, however subtle hormonal hypersecretion not leading to the full clinical phenotype of a related syndrome may be present in patients with an AI (6).
Screening for Cortisol Excess
According to the Endocrine Society’s Clinical Practice Guidelines for the diagnosis of Cushing’s syndrome and the AACE/AAES Medical Guidelines for the management of AIs, all patients with an incidentally discovered adrenal mass should be tested for the presence of hypercortisolism (64,98). Signs and symptoms of overt Cushing’s syndrome if present in a thorough clinical evaluation should prompt the physician to proceed with the recommended diagnostic approach described in the relevant Endocrine Society’s Clinical Guidelines (98). In this case, as discussed earlier, the validity of the term “incidentaloma” is debated.
In the absence of overt disease, biochemical investigation frequently reveals subtle cortisol hypersecretion and abnormalities of the HPA axis, a state previously termed as subclinical Cushing’s syndrome (6). Based on the most recent clinical practice guidelines by the European Society of Endocrinology (ESE) and European Network for the Study of Adrenal Tumors (ENSAT) the term “mild autonomous cortisol secretion” (MACS) is preferred and will also be used throughout this chapter. Although MACS is poorly defined, and its natural history is unclear (3), the prevalence of hypertension, diabetes, obesity, other features of the metabolic syndrome, and osteoporosis has been found to be increased in such patients (5,99). Because standard biochemical tests used to screen for Cushing’s syndrome were not designed to reveal the subtle changes encountered in MACS, and since a definitive clinical phenotype to ascertain the presence of this condition is missing, a combination of various parameters used to assess the integrity of the HPA axis have been employed. Alterations of the HPA axis suggestive of MACS in AIs include altered dexamethasone suppression (DST) and response to CRH, increased mean serum cortisol and urinary free cortisol (UFC) levels, reduced dehydroepiandrosterone sulfate (DHEA-S) and reduced ACTH levels (33), although the latter has recently been questioned since most ACTH assays lack sensitivity at the lower part of the reference range (100). Incorporation of midnight salivary cortisol as a means to diagnose MACS has produced inconsistent results (101).
Currently, the 1 mg overnight DST, remains the most reliable and easily reproducible method and is the recommended test to detect cortisol secretion abnormalities based on pathophysiological reasoning, simplicity, and incorporation in the diagnostic algorithms of most studies. (5,101). Cortisol autonomy in AIs reflects a biological continuum without a clear separation between functioning and non-functioning tumors. Different cortisol cut-off values following the 1 mg DST have been advocated from different authors and were adopted by several authorities, ranging from 50 to 138 nmol/l (1.8 to 5 μg/dl) (64,102). Higher thresholds increase the specificity of the test but lower its sensitivity (103). The post 1 mg DST cortisol cutoff of >5 μg/dl (138 nmol/l) approach was substantiated by studies showing that all patients with such a cortisol value had uptake only on the side of the adenoma on adrenal scintigraphy (104). On the other hand, studies that used post-surgical hypoadrenalism as indicative of autonomous cortisol secretion suggested that lower cortisol cut-offs may be needed to identify these cases (105–107). Furthermore, older stratification of autonomy based on different post-1mg ODST cortisol levels has been abandoned by recent guidelines (66). A negative DST using a cortisol cut-off value of 1.8 μg/dl (50 nmol/l) virtually excludes MACS. Furthermore, several studies have found that patients with post DST cortisol values >1.8 μg/dl (50 nmol/l) have increased morbidity or mortality (108,109) .The formal low dose dexamethasone suppression test (LDDST) can be used to confirm and quantify the degree of autonomous cortisol secretion or to exclude a false positive test (110,111). Based on our experience, the post-LDDST cortisol value should be considered in patients with such intermediate cortisol values following the 1 mg DST because, in addition to its high specificity, it correlates well with other indices of cortisol excess and the size of the adenoma, thus providing a quantitative measure of the degree of cortisol production from the adenoma and a more robust means for further follow-up (110,112). Although confirmation of ACTH independency (through suppressed ACTH levels) is also required to establish the diagnosis of MACS (64), the 1 mg DST should be the initial screening test based on pathophysiology and the fact that it represents the most common HPA axis abnormality reported by most studies (49). It should also be noted that cortisol levels after 1mg DST are increasing with age, making the diagnosis of MACS in frail elderly patients difficult. Especially for this subgroup of patients in which comorbidities are already frequently present, MACS diagnosis is not considered clinically relevant, and could be omitted. Finally, it is important to consider drugs or conditions that interfere with this test by altering dexamethasone absorption, metabolism by CYP3A4, or falsely elevate cortisol levels through increased cortisol-binding globulin (CBG) levels (113). Consequently, repeating the 1mg overnight DST in patients who were previously tested positive, and especially those who are candidates for surgery, is advisable.
Reduced levels of DHEA-S also reflect chronic suppression of ACTH secretion and have been found to offer comparable sensitivity and greater specificity to the existing gold-standard 1 mg DST for the diagnosis of MACS (114). In a study of 185 patients with AIs of which 29 patients (16%) were diagnosed with autonomous cortisol secretion, an age- and sex-specific DHEA-S ratio (derived by dividing the DHEA-S by the lower limit of the respective reference range for age and sex) of <1.12 was >99% sensitive and 92% specific for the diagnosis of MACS (115). In a retrospective study of 256 patients with AIs and MACS, a serum DHEA-S concentration <40 μg/dL was 84% specific for MACS, whereas an ACTH concentration <10 pg/mL was only 75% specific for MACS. In addition, a serum concentration of DHEAS >100 μg/dL combined with an ACTH >15 pg/mL was 96% percent specific for excluding MACS (116). The only caveat is that age- and sex- adjusted DHEA-S reference values are not well established.
Recently, studies utilizing gas chromatography-tandem mass spectrometry (GC-MS/MS) to measure serum and 24-hour urine levels of several steroids in patients with AIs have emerged, showing promising potential. Patients with MACS have been found to have decreased levels of adrenal androgens and their metabolites and increased levels of glucocorticoid metabolites compared to healthy individuals, with sensitivity and specificity rates comparable to routine methods (117–119).
Since cortisol-related comorbidities play such an important role in planning patient management, it is crucial to gather medical information and laboratory data about glucose and lipid metabolism, hypertension, bone density and fractures.
Screening for Pheochromocytoma
Although arterial hypertension and other signs of catecholamine excess are considered classical clinical manifestations of PCCs, screening should be performed even in normotensive patients with AIs since catecholamine secretion can be intermittent, and cases of “silent” PCCs are increasingly being recognized (120). The initial recommended biochemical screening test is measurement of plasma free (from blood drawn in the supine position) or urinary fractionated metanephrines using liquid chromatography with mass spectrometric or electrochemical detection methods (40). This approach has a sensitivity and specificity of 99% and 97% respectively and has proven to be superior to measurement of plasma or urine catecholamines and vanillylmandelic acid (VMA) (121). The issue concerning the diagnostic performance of plasma free versus urinary fractionated metanephrines has been recently settled in a multicenter prospective study involving over 2,000 patients, with follow-up to exclude patients without PPGL and with LC-MS/MS measurements of plasma and urinary free metanephrines compared to urinary deconjugated metanephrines (122). In this study, diagnosis of PPGLs using plasma or urinary free metabolites provided advantages of fewer false-positive results compared with commonly measured de-conjugated metabolites. The plasma panel offered better diagnostic performance than either urinary panel for high-risk patients but was comparable for patients at low risk of disease. Sane et al suggested that routine biochemical screening for PCC in small (<2cm) homogenous AIs characterized by attenuation values <10 HU may not be necessary, since none of the 115 patients in his cohort with lipid-rich tumors (<10 HU) had constantly elevated 24-hour urinary metanephrines or normetanephrines, whereas all 10 histologically proven PCCs were larger than 2cm and were characterized by >10 HU in unenhanced CT scans (123). This was also confirmed from a recent multicenter retrospective study including 376 PCCs with sufficient data from CT imaging. Based on the lack of PCCs with an unenhanced attenuation of <10 HU and the low proportion (0.5%, 2/376) of PCCs with an attenuation of 10 HU, it was suggested that abstaining from biochemical testing for PCC in AIs with an unenhanced attenuation of ≤10 HU is reasonable, whereas contrast washout measurements were unreliable for ruling out PCC (124).
A recent study (125) comparing the clinical, hormonal, histological, and molecular features of normotensive incidentally discovered PCCs (previously referred as “silent”) with tumors causing overt symptoms, revealed lower diagnostic sensitivity (75%) for plasma and urinary metanephrines irrespective of tumor size, while genetic and histological studies showed decreased expression of genes and proteins associated with catecholamine production and increased cellularity and mitotic activity in “silent” tumors. It was implied that asymptomatic incidentally discovered PCCs do not represent an early stage of development of PCCs but rather correspond to a distinct entity characterized by cellular defects in chromaffin machinery resulting in lower efficiency to produce or release catecholamines. It is, therefore, crucial to consider that normotensive patients with an AI and normal values of metanephrines, may indeed harbor a PCC. In such instance, the CT and MRI scan features of the tumor if suspicious for PCC, should alert the clinician to perform complementary investigations, such as plasma chromogranin A measurement, MIBG scintigraphy, 18F-FDG-PET/CT, or other alternative functional imaging (F-DOPA/PET or FDA/PET) to rule out this possibility.
Screening for Aldosterone Excess
According to published guidelines from the Endocrine Society, all patients with an AI and hypertension, irrespective of serum potassium levels, should be tested for PA using the plasma aldosterone/renin ratio (ARR) as a screening test (42). However, the knowledge that PA can be diagnosed in normotensive patients with hypokalemia necessitates testing of all patients with hypertension or hypokalemia (44). Although there is no current consensus regarding the most diagnostic ARR cut-off, values >20-40 (plasma aldosterone expressed as ng/dl and plasma renin activity [PRA] as ng/ml/h) obtained in the morning from a seated patient are highly suggestive. However, the plasma aldosterone level also needs to be considered because extremely low PRA, even in the presence of normal aldosterone levels, will result in a high ARR; an aldosterone level less than 9 ng/dl makes the diagnosis of PA unlikely, whereas a level in excess of 15 ng/dl is suggestive (49). Attention should also be given in certain technical aspects required for the prompt interpretation of the ARR such as unrestricted dietary salt intake, corrected potassium levels, and washout of interfering antihypertensive medication. Patients may be treated with a non-dihydropyridine calcium channel blocker (verapamil slow release) as a single agent or in combination with α-adrenergic blockers (such as doxazosin) and hydralazine for blood pressure control during the washout period, if needed.
When suspected based on the ARR, PA should be verified with one of the commonly used confirmatory tests (oral sodium loading, saline infusion, fludrocortisone suppression, and captopril challenge). Admittedly, the extent that patients with AI should be investigated to exclude PA is still not known. Although PA has been reported with a low prevalence between patients with AIs (1-10%), substantially higher rates (24%) have recently been described using a recumbent post-low dose dexamethasone suppression (LDDST)-saline infusion test (PD-SIT) (45). Further studies evaluating the optimal biochemical diagnostic approach of PA in patients with AIs are required by comparing established versus evolving investigational protocols.
Screening for Androgen/Estrogen Excess
Measurement of sex hormones is not recommended in patients with an AI on a routine basis (64). Elevated levels of serum DHEA-S, androstenedione, 17-OH progesterone as well as testosterone in women and estradiol in men and postmenopausal women can be found in more than half of patients with ACCs (126). Although cases of androgen or estrogen excess have been rarely described in patients with benign adrenocortical adenomas (127–130), they are usually accompanied by symptoms or signs of virilization in women (acne, hirsutism) or feminization in men (gynecomastia), and therefore such lesions cannot be considered as true AIs. Thus, the usefulness of measuring sex hormones and steroid precursors is limited to cases of adrenal lesions with indeterminate or suspicious for malignancy imaging characteristics, where elevated levels can point towards the adrenocortical origin of the tumor and suggest the presence of an ACC rather than a metastatic lesion. Additionally, increased basal or after cosyntropin stimulation levels of 17-OH progesterone can also indicate CAH in patients with bilateral AIs (6).
Screening for Hypoadrenalism
Bilateral AIs caused by metastases of extra-adrenal malignancies or infiltrative diseases can rarely cause adrenal insufficiency (131). Therefore, in all patients with bilateral adrenal masses, adrenal insufficiency should be considered and evaluated clinically and if likely, diagnosis should be established using the standard 250μg cosyntropin stimulation test according to the Endocrine Society’s recently published clinical guidelines (132).
FINE NEEDLE ASPIRATION BIOPSY (FNAB)
Percutaneous fine-needle aspiration biopsy (FNAB) as means to clarify the nature of an AI has now been surpassed by the non-invasive radiological methods because they have better diagnostic accuracy and are devoid of potential side effects (133,134). It should be noted that FNAB is not considered an accurate method in differentiating benign from malignant primary adrenal tumors (135) but can be helpful in the diagnosis of metastases from extra-adrenal malignancies, lymphoma, sarcoma, infiltrative or infectious process with a sensitivity of 73-100% and a specificity of 86-100% using variable population inclusion criteria, reference standards, and biopsy techniques (136–138). Adrenal biopsy is not needed if the patient is already known to have widespread metastatic disease. Biopsy is only recommended for hormonally inactive masses not characterized as benign on imaging and where a biopsy result would affect treatment decisions. FNAB has significant procedural risk with complications such as pneumothorax, bleeding, infection, pancreatitis, and dissemination of tumor cells along the needle track reported at a rate up to 14% by some, but not all available studies (133). To avoid the risk of a potentially lethal hypertensive crisis, PCC should always be excluded biochemically before FNA of an adrenal mass is attempted (139).
NATURAL HISTORY OF AIs
Since AIs do not represent a single clinical entity, their natural history varies depending on the underlying etiology. Primary malignant adrenal tumors typically display rapid growth (>2 cm/year) and a poor outcome with an overall 5-year survival of 47%. It is not known whether prognosis of patients with incidentally discovered ACC is different from symptomatic cases, however detection of the tumor at an early stage provides the possibility of definitive surgical cure (140). Patients bearing adrenal metastases have a clinical course depending on stage, grade, and site of the primary tumor (4). PCCs grow slowly and are mostly benign, but if untreated are potentially lethal displaying high cardiovascular mortality and morbidity, whereas 10-17% of the cases can be malignant (40). This is further emphasized by the fact that PCCs detected in autopsy series had not been suspected in 75% of the patients while they were alive, although they contributed to their death in approximately 55% of cases (141).
In benign adrenal tumors, which constitute the majority of AIs, the main concerns about their natural history revolve around their progressive growth, the possibility of malignant transformation, and the risk of evolution towards overt hypersecretion. Several cohort studies, despite their limitations, have shown that the majority of benign tumors remain stable in size; only 5-20% show a >1 cm increase in size, mostly within the first three years after prolonged follow-up (142,143), whereas occasional shrinkage, or even complete disappearance, of an adrenal mass have also been reported in about 4% of cases (8,144). Although there is not a specific growth rate cut-off indicative of a benign nature, ACCs initially presenting as AIs, are invariably characterized by a rapid growth within months (at least > 0.8cm/year). The risk of an AI initially considered to be benign to become malignant has been estimated at <1/1000 (3,8) by Cawood et al, who found only two reports of a malignancy detected during the follow-up of AIs presenting as benign at diagnosis; the first was a renal carcinoma metastasis in a patient with a known history of renal carcinoma and the other was a non-Hodgkin’s lymphoma that showed a mass enlargement after 6 months (3). Two case reports of patients with a well-documented history of adrenal incidentalomas with totally benign imaging features on CT, who were diagnosed on follow-up (8 and 14 years later) with a malignant tumor in the same adrenal gland have recently been described (145,146). It is not known whether these cases can be explained by the independent occurrence of two events in a single adrenal (initially a typical benign adenoma and consequently the occurrence of an ACC) or whether a malignant transformation of a benign adenoma to carcinoma was the underlying course of events. Although there is evidence to suggest the adenoma-carcinoma sequence is possible in the adrenal cortex (147,148), the high prevalence of adenomas contrasting with the extremely low prevalence of ACCs suggest that this process is probably exceptionally rare. These findings highlight the low risk of malignant transformation of AIs and the adequacy of current imaging to ascertain the diagnosis at presentation deterring the need for long-term imaging follow-up.
The appearance of hormonal hypersecretion over time in initially NFAIs varies in different series. New-onset catecholamine or aldosterone overproduction is extremely rare (<0.3%), whereas development of overt hypercortisolism during follow-up is found in <1% (8). The most common disorder observed during follow-up is the occurrence of autonomous cortisol secretion eventually leading to MACS, reported with a frequency of 5.4% (CI 3,1-8,1%) (66,144). This risk is higher for lesions >3 cm in size and during the first 2 years of follow-up but seems to plateau after 3-4 years, even if it does not subside completely (149). On the other hand, subtle hormonal alterations discovered at initial screening may also improve over time, indicating possible cyclical cortisol secretion from AIs and/or highlighting the inherent difficulty in biochemical confirmation of this condition (143).
Another issue of debate regarding the natural history of AIs that has attracted research, producing frequently conflicting data, is the sequelae of MACS on cardiovascular risk and subsequent mortality and morbidity. Several cross-sectional and cohort studies have reported a clustering of unfavorable cardiovascular risk factors in patients with AIs similar to those found in patients with overt Cushing’s syndrome (150,151). It is biologically plausible to anticipate that the presence of even mild to minimal cortisol excess may lead to some extent to the classic long-term consequences of overt hypercortisolism, such as hypertension, obesity, impaired glucose tolerance or frank diabetes, dyslipidemia, and osteoporosis (figure 4). Because these metabolic derangements are common in the general and particularly the elderly population, in whom AIs are more frequently found, it is difficult to extrapolate whether there is a causal relationship between them. Whether these metabolic abnormalities in patients with AIs result in increased cardiovascular mortality and morbidity has not as yet been fully clarified. Although, some recent retrospective studies (108,109,152,153) have shown higher rates of cardiovascular events and mortality in patients with higher cortisol levels after the 1 mg DST, data from patients who underwent adrenalectomy are contradictory, regarding the outcome on metabolic and cardiovascular profile, whereas there are relatively few data on the risk of major cardiovascular events or mortality (107,154–156). Similarly, evidence on the detrimental effects of MACS on bone metabolism, such as lower bone density and high prevalence of vertebral fractures (43-72%) in postmenopausal women and eugonadal male patients with AIs (99,157–160) are conflicting with studies not showing reversal of these effects following surgical treatment (154,161). Additionally, most of the detected vertebral fractures were minor and of uncertain clinical impact (99).
Moreover, there is growing evidence that even non-functioning Ais (NFAIs) may be associated with similar metabolic disturbances and manifestations of the metabolic syndrome that are considered cardiovascular risk factors (162–164). Compared with controls, patients with NFAIs exhibit subtle indices of atherosclerosis such as increased carotid intima-media thickness (IMT)(165), impaired flow-mediated vasodilatation (FMD) (166), and left ventricular hypertrophy (167). A recent study excluding patients with traditional risk factors (diabetes, hypertension or dyslipidemia) reported similar findings in patients harboring NFAIs, with increased insulin resistance and endothelial dysfunction that correlated with subtle but not autonomous cortisol excess (168). Furthermore, an observational study suggested that patients with NFAIs had a significantly higher risk of developing diabetes compared with control subjects without adrenal tumors prompting a re-assessment of whether the classification of benign adrenal tumors as “non-functional” adequately reflects the continuum of hormone secretion and metabolic risk they may harbor (169).
A recent meta-analysis (170) of 32 studies including patients with NFAIs and adrenal tumors associated with MACS provided important insights on the natural history of such tumors that help in solving controversy and informing practice. First and foremost, it was observed that only a small proportion of patients with NFAI or MACS had tumor growth or changes in hormone production during follow-up. Only 2.5% of adrenal incidentalomas grew by 10 mm or more over a mean follow-up of 41.5 months, whereas the mean difference in adenoma size between follow-up and baseline in all patients was negligible at 2.0 mm. Larger adenomas at diagnosis (≥25 mm) were even less likely than smaller tumors to grow during follow-up, which, according to the authors, suggests attainment of maximum growth potential. More importantly malignant transformation was never observed at the end of follow-up. Similarly, in patients with NFAIs or MACS at diagnosis, the risk of developing clinically overt hormonal hypersecretion syndromes (Cushing’s, PA, or catecholamine excess) was negligible (<0,1%), suggesting that these rare cases are probably attributed to the development of subsequent adrenal tumors and that MACS does not represent a preliminary stage of overt Cushing’s. Inapparent cortisol autonomy ensued only in 4.3% of patients with initially nonfunctioning tumors. The third and most novel finding of this thorough meta-analysis pertained to comorbidities, cardiovascular risk, and mortality. It was confirmed, like in other similar studies, that patients with MACS had a high prevalence of cardiovascular risk factors (such as hypertension, obesity, dyslipidemia, and type 2 diabetes) and were more likely than those with NFAIs to develop or show worsening of these factors during follow-up. However, the prevalence of such factors in patients with NFAIs was also significant and higher than expected for Western populations. This finding could be explained by a subtle degree of glucocorticoid excess not detected by current diagnostic criteria or perhaps by cyclical cortisol secretion or even by excess cortisol secretion in response to stress situations. It could also represent ascertainment bias since patients with diseases are more likely to have imaging tests that may detect an AI or could be a result of the previously theorized reverse causality concept that diabetes or the metabolic syndrome promote adrenal tumor development (171). Interestingly, reported all-cause and cardiovascular mortality in patients with NFAI during follow-up were similar to those in patients with MACS, warranting close clinical follow-up and treatment for both groups of patients.
MANAGEMENT
A proposed algorithm for diagnostic approach and management of AIs based on the more recently published and widely accepted guidelines (66) is presented in Figures 4 and 5. A patient presenting with a newly discovered AI should be initially assessed in parallel for its malignancy potential and functional status. Exclusion of malignancy is critical and imaging review by an experienced radiologist is of crucial importance. Since evidence for the accuracy of MRI-CSI is not as strong, non-contrast CT is the first modality that should be used if not already performed. An unenhanced attenuation value of ≤ 10 HU combined with homogeneity can safely, based on available data, confirm the diagnosis of benign adenoma and exclude malignancy, requiring no further imaging investigation or follow-up. The same can be applied for larger AIs (>4cm) with unequivocal benign phenotype (≤ 10 HU, homogeneous), since recent observational data have provided better quality evidence for their benign natural course (72,172). For tumors with >10 HU, management is dependent on the risk of malignancy based on a combination of imaging properties such as attenuation (11-20 HU or >20 HU), size (< or > 4cm) and homogeneity (homogeneous or heterogeneous). In a homogeneous, < 4 cm adrenal mass with unenhanced HU between 11 and 20, the likelihood of malignancy is <10%. Thus, the proposed approach is to immediately acquire an additional imaging study, depending on the local experience and preference (FDG-PET/CT, MRI with CSI or CT with washout protocol). If the findings from the additional imaging are suggestive of a benign lesion, no further imaging follow-up is required. Alternatively, interval imaging (with non-contrast CT or MRI) after 12 months could be performed, to ensure that no significant change in size has occurred. On the opposite side, AIs that have relatively high risk of malignancy should be discussed in a multidisciplinary team (MDT) meeting. Those include AIs ≥4 cm with density > 20 HU or a heterogeneous appearance and are most likely candidates for immediate surgical removal. Prior to surgery staging with chest CT and/or FDG/PET-CT is recommended to detect metastatic disease if present. In case the MDT recommendation is not surgery, interval imaging (with non-contrast CT or MRI) in 6-12 months is advised. All other AIs with intermediate tumor characteristics (tumor size ≥ 4 cm with unenhanced HU 11-20, or tumor size < 4 cm with unenhanced HU > 20, or tumor size < 4 cm with heterogeneous appearance), have a smaller but considerable relative risk for malignancy and should be examined in detail in an MDT meeting. Ordering additional imaging (FDG-PET/CT, MRI with CSI or CT with washout protocol, depending on local availability and expertise) seems to be the appropriate strategy. In these cases, additional imaging with FDG/PET-CT might have an advantage over the other modalities due to the low risk of false negative results. If the tumor remains indeterminate after the additional imaging workup, surgery or interval imaging (with non-contrast CT or MRI) after 6-12 months could be offered. A promising alternative to additional imaging, that has appeared in recent years, is urine or plasma steroid metabolomics (profiling) by tandem mass spectrometry. In two published retrospective studies (72,119), one using urine and the other plasma samples, sensitivity for excluding adrenocortical cancer, as stand-alone tests, was approximately 80%. However, when combined with imaging properties (namely attenuation >20 HU and size >4cm) urine steroid metabolomics showed a negative predictive value of 99.7%.
Interval imaging at 6 and/or 12 months in case no surgery is performed (MDT decision or for any other reason) is done to monitor possible progressive growth. An increase of >20% of the largest tumor diameter together with an at least 5 mm increase in this diameter (101), as defined by RECIST 1.1 criteria, or an absolute increase by >8 mm over 12 months, as suggested by some studies (67), probably warrant re-evaluation by the MDT. Further imaging follow-up may not be needed if no change is size is seen at the first interval imaging.
In indeterminate cases, age is a parameter that needs to be considered by the MDT when deciding which patients to refer for adrenalectomy. For example, most clinicians would tend to advise in favor of removing a lipid-poor (19 HU) 3.2 cm AI in a 23-year-old woman, whereas serial imaging follow-up would be favorably recommended in an 83-year-old woman with a lipid-poor (15 HU) 4.7 cm adrenal tumor.
All published guidelines and expert reviews agree that patients with unilateral adrenal masses causing unambiguous hormonal overactivity, and those with suspected malignancy (mainly ACC), are candidates for surgical interventions (5,6,40,42,64,66,101,102,173,174). There is also broad consensus that the majority of AIs with clearly benign imaging phenotype in unenhanced CT and no evidence of functionality do not require surgery.
The management of patients harboring AIs who have MACS is debatable and the beneficial effect of adrenalectomy has not been proven adequately in the literature. Some, but not all, predominantly retrospective studies have shown a beneficial effect in hypertension and diabetes mellitus in patients with AIs who underwent an adrenalectomy, compared to those who did not undergo such a procedure (107,154,156). In one prospective study with an 8-year follow-up, operated patients with MACS had an improvement in features of the metabolic syndrome, but not of osteoporosis, compared to those who were conservatively managed; however, no control group was included in the study (154). An improvement of blood pressure and blood glucose was noted in a retrospective study of adrenalectomized patients with MACS, whereas these indices worsened in non-operated patients; even so, some patients apparently with NFAI also showed an improvement in some of these parameters (107). In a recent prospective multicenter randomized study including 62 patients aged 40-75, Morelli et al showed that adrenalectomy more frequently ameliorated hypertension (68% versus 13%) and glycometabolic control (28% versus 3,3%) than the conservative approach, while the latter was associated with a more frequent worsening of blood pressure and insulin resistance (12% versus 40%). Since available data from the aforementioned retrospective and the two recent small prospective studies are not considered high-quality, the decision to recommend surgery should be taken in a multidisciplinary setting while taking several other factors into consideration, such as: duration and evolution of comorbidities and their degree of control, presence and extent of end organ damage inappropriate for age, discrepant family history, presence of multiple comorbidities, age, sex, general health, degree and persistence of nonsuppressible cortisol after dexamethasone, and patient’s preference. Young patients with MACS and those with new onset and/or rapidly worsening comorbidities resistant to medical treatment (6,175) could thus be candidates for surgical intervention.
Myelolipomas are considered benign tumors, their diagnosis is mostly based on imaging characteristics and biochemical evaluation is not usually needed unless informed by clinical presentation. Measurement of 17(OH) progesterone is advised in large and/or bilateral myelolipomas for the possibility of CAH. Their management is mostly conservative with yearly imaging follow-up, since in up to 16% of the cases a median tumor growth of at least 1cm per year was demonstrated. Surgery is usually reserved for large tumors, those with tumor growth, acute hemorrhage, symptoms of abdominal mass effect, or uncontrolled CAH (176).
Before proceeding to surgical therapy, appropriate medical therapy must be given to all functioning lesions, aiming at symptom control. Apart from patients with Cushing’s syndrome, post-surgical adrenal insufficiency may ensue in MACS patients (177,178). Because the need for glucocorticoid coverage cannot be predicted before surgery, patients should be covered by steroids post-operatively until the HPA-axis can be formally assessed (105). Low morning cortisol levels the day after surgery, and before glucocorticoid replacement, provide evidence for post-surgical hypoadrenalism (107). All patients diagnosed with PCC, including normotensive patients with “silent” tumors should receive preoperative α-adrenergic blockade for 7 to 14 days to prevent perioperative cardiovascular complications. Treatment should also include a high-sodium diet and fluid intake to reverse catecholamine-induced blood volume contraction preoperatively and prevent severe hypotension after tumor removal (40). Finally, patients diagnosed with PA and bilateral tumors or a unilateral AI (if older than 40 years of age) who seek a potential surgical cure, should be considered for adrenal venous sampling (AVS) before proceeding to surgery, to confirm lateralization of the source of the excessive aldosterone secretion. In cases where decision for adrenalectomy is based on imaging phenotype it would also be prudent to exclude the possibility of a “silent” PCC before proceeding to surgery, because hemodynamic instability during surgical excision may ensue.
According to earlier published AACE/AAES Medical Guidelines for the management of adrenal incidentalomas, patients with AIs not elected for surgery after the initial diagnostic work-up, should undergo re-imaging 3-6 months after the initial diagnosis and then annually for the next 1-2 years, while annual biochemical testing is advised for up to 4-5 years following the diagnosis (64). However, it has recently been suggested by some authors that given the low probability of the transformation of a benign and non-functioning adrenal mass to a malignant or functioning one, the routine application of the current strategies in all patients with AIs is likely to result in a number of unnecessary biochemical and radiological investigations (3,179,180). Such an approach is costly, and it does not take into account harmful consequences of diagnostic evaluation such as patients’ anxiety associated with repeated clinical visits and a high rate of false positive results leading to further testing or unnecessary adrenalectomy. Moreover, exposure to ionizing radiation from repeated CT scans increases the future cancer risk to the level that is similar to the risk of the adrenal lesion becoming malignant (3,181).
Patients without any biochemical abnormalities at presentation could be spared the burden of repeated testing, since the risk of developing clinically overt hormonal excess is extremely low. Clinical follow-up with assessment of cardiovascular risk factors that have been associated with the presence of AIs may be adequate to detect the reported ~10% of the cases of new onset MACS (5). Patients with worsening of their metabolic parameters should be retested with the 1mg DST and be advised to apply lifestyle changes and effective medical treatment to reduce cardiovascular risk. If biochemical abnormalities suggesting MACS are present during the initial screening, annual clinical follow-up including evaluation of potentially cortisol excess-related comorbidities, as well as periodic testing of the HPA axis, is advisable. Patients with MACS who do not reach the treatment goals despite an adequate medical therapy could be offered surgery. Duration of follow-up is also under debate, however based on available data, annual hormonal evaluation may be suggested for up to five years, and especially for lesions >3 cm (64).
CONCLUSION
AIs are increasingly being recognized, particularly in the aging population. Adrenal CT and MRI can reliably distinguish benign lesions, while 18F-FDG-PET/CT scan can be helpful in identifying tumors with malignant potential. MACS is the most common hyperfunctional state that is best substantiated using the 1 mg DST; urinary/plasma metanephrines and ARR are used to screen for PCCs and hyperaldosteronism. Adrenal lesions with suspicious radiological findings, PCCs and tumors causing overt clinical syndromes, as well as those with considerable growth during follow-up, should be treated with surgical resection. Although there is no consensus, the interval for diagnostic follow-up testing relies on the radiological and hormonal features of the tumors at presentation. The benefit of surgical resection in patients with substantial comorbidities and associated subclinical adrenal hyperfunction, mainly in the form of MACS, is still under investigation.
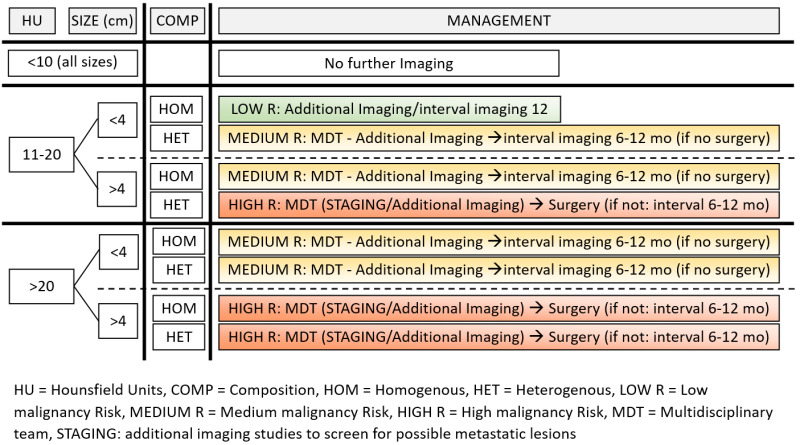
Figure 4.
Proposed algorithm for diagnosis and management of AIs (imaging evaluation).
REFERENCES
- 1.
- Korobkin M, White E, Kressel H, Moss A, Montagne J. Computed tomography in the diagnosis of adrenal disease. American Journal of Roentgenology 1979;132(2):231–238. [PubMed: 105590]
- 2.
- Vassiliadi D a, Tsagarakis S. Endocrine incidentalomas--challenges imposed by incidentally discovered lesions. Nat Rev Endocrinol 2011;7(11):668–80. [PubMed: 21709710]
- 3.
- Cawood TJ, Hunt PJ, O'Shea D, Cole D, Soule S. Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant; time for a rethink? Eur J Endocrinol 2009;161(4):513–527. [PubMed: 19439510]
- 4.
- Grumbach MM, Biller BMK, Braunstein GD, Campbell KK, Aidan Carney J, Godley PA, Harris EL, Lee JKT, Oertel YC, Posner MC, Schlechte JA, Wieand S, Marciel K, Carney JA, Godley PA, Harris EL, Lee JKT, Oertel YC, Posner MC, Schlechte JA, Wieand HS. Management of the clinically inapparent adrenal mass (“incidentaloma”). In: Annals of Internal Medicine.Vol 138.; 2003:424–429. [PubMed: 12614096]
- 5.
- Terzolo M, Stigliano A, Chiodini I, Loli P, Furlani L, Arnaldi G, Reimondo G, Pia A, Toscano V, Zini M, Borretta G, Papini E, Garofalo P, Allolio B, Dupas B, Mantero F, Tabarin A. AME position statement on adrenal incidentaloma. Eur J Endocrinol 2011;164(6):851–870. [PubMed: 21471169]
- 6.
- Young WF. The Incidentally Discovered Adrenal Mass. New England Journal of Medicine 2007;356(6):601–610. [PubMed: 17287480]
- 7.
- Nawar R. Adrenal incidentalomas -- a continuing management dilemma. Endocrine Related Cancer 2005;12(3):585–598. [PubMed: 16172193]
- 8.
- Barzon L, Sonino N, Fallo F, Palù G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol 2003;149(4):273–285. [PubMed: 14514341]
- 9.
- Ebbehoj A, Li D, Kaur RJ, Zhang C, Singh S, Li T, Atkinson E, Achenbach S, Khosla S, Arlt W, Young WF, Rocca WA, Bancos I. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: a population-based cohort study. Lancet Diabetes Endocrinol 2020;8(11):894–902. [PMC free article: PMC7601441] [PubMed: 33065059]
- 10.
- van den Broek J, Geenen R, Heijnen L, Kobus C, Schreurs H. Adrenal Incidentalomas During Diagnostic Work-up of Colorectal Cancer Patients: What is the Risk of Metastases? Ann Surg Oncol 2018;25(7):1986–1991. [PubMed: 29761333]
- 11.
- Rineheart JF WO and CW. Adenomatous hyperplasia of the adrenal cortex associated with essential hypertension. Arch Pathol (Chic) 1941;(34):1031–1034.
- 12.
- RUSSI S, BLUMENTHAL HT, GRAY SH. Small adenomas of the adrenal cortex in hypertension and diabetes. Arch Intern Med (Chic) 1945;76:284–91. [PubMed: 21011882]
- 13.
- COMMONS RR, CALLAWAY CP. Adenomas of the adrenal cortex. Arch Intern Med (Chic) 1948;81(1):37–41. [PubMed: 18899021]
- 14.
- Schroeder H. Clinical types - the endocrine hypertensive syndrome. In: Schroeder H, ed. Hypertensive Diseases: Causes and Control. Philadelphia: Lea & Febiger; 1953:295–333.
- 15.
- Dévényi I. Possibility of normokalaemic primary aldosteronism as reflected in the frequency of adrenal cortical adenomas. J Clin Pathol 1967;20(1):49 LP – 51. [PMC free article: PMC473419] [PubMed: 6016887]
- 16.
- Salomon MI, Tchertkoff V. Adrenal adenoma and hypertension. Ann Intern Med 1972;76(4):668–669. [PubMed: 4640328]
- 17.
- Hedeland H, Östberg G, Hökfelt B. ON THE PREVALENCE OF ADRENOCORTICAL ADENOMAS IN AN AUTOPSY MATERIAL IN RELATION TO HYPERTENSION AND DIABETES. Acta Med Scand 1968;184(1‐6):211–214. [PubMed: 5703975]
- 18.
- Yamada EY, Fukunaga FH. Adrenal Adenoma and Hypertension a Study in the Japanese in Hawaii. Jpn Heart J 1969;10(1):11–19. [PubMed: 5305451]
- 19.
- Granger P, Genest J. Autopsy study of adrenals in unselected normotensive and hypertensive patients. Can Med Assoc J 1970;103(1):34–36. [PMC free article: PMC1930349] [PubMed: 5424294]
- 20.
- Russell RP, Masi AT, Richter ED. Adrenal cortical adenomas and hypertension: A clinical pathologic analysis of 690 cases with matched contbols and a review of the literature. Medicine (United States) 1972;51(3):211–225. [PubMed: 5021770]
- 21.
- Abecassis M, McLoughlin MJ, Langer B, Kudlow JE. Serendipitous adrenal masses: Prevalence, significance, and management. The American Journal of Surgery 1985;149(6):783–788. [PubMed: 4014556]
- 22.
- Meagher AP, Hugh TB, Casey JH, Chisholm DJ, Farrell JC, Yeates M. PRIMARY ADRENAL TUMOURS – A TEN‐YEAR EXPERIENCE. Australian and New Zealand Journal of Surgery 1988;58(6):457–462. [PubMed: 3270316]
- 23.
- Reinhard C, Saeger W, Schubert B. Adrenocortical nodules in post-mortem series. Development, functional significance, and differentiation from adenomas. Gen Diagn Pathol 1995;141(3–4):203–208. [PubMed: 8705784]
- 24.
- Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev 1995;16(4):460–484. [PubMed: 8521790]
- 25.
- Masumori N, Adachi H, Noda Y, Tsukamoto T. Detection of adrenal and retroperitoneal masses in a general health examination system. Urology 1998;52(4):572–576. [PubMed: 9763073]
- 26.
- Glazer H, Weyman P, Sagel S, Levitt R, McClennan B. Nonfunctioning adrenal masses: incidental discovery on computed tomography. American Journal of Roentgenology 1982;139(1):81–85. [PubMed: 6979870]
- 27.
- Prinz RA, Brooks MH, Churchill R, Graner JL, Lawrence AM, Paloyan E, Sparagana M. Incidental Asymptomatic Adrenal Masses Detected by Computed Tomographic Scanning: Is Operation Required? JAMA: The Journal of the American Medical Association 1982;248(6):701–704. [PubMed: 7097921]
- 28.
- Belldegrun A, Hussain S, Seltzer SE, Loughlin KR, Gittes RF, Richie JP. Incidentally discovered mass of the adrenal gland. Surg Gynecol Obstet 1986;163(3):203–208. [PubMed: 3750174]
- 29.
- Herrera MF, Grant CS, van Heerden JA, Sheedy PF, Ilstrup DM. Incidentally discovered adrenal tumors: an institutional perspective. Surgery 1991;110(6):1014–21. [PubMed: 1745970]
- 30.
- Caplan RH, Strutt PJ, Wickus GG. Subclinical Hormone Secretion by Incidentally Discovered Adrenal Masses. Archives of Surgery 1994;129(3):291–296. [PubMed: 8129606]
- 31.
- Bovio S, Cataldi A, Reimondo G, Sperone P, Novello S, Berruti A, Borasio P, Fava C, Dogliotti L, Scagliotti G V., Angeli A, Terzolo M. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest 2006;29(4):298–302. [PubMed: 16699294]
- 32.
- Song JH, Chaudhry FS, Mayo-Smith WW. The incidental adrenal mass on CT: Prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. American Journal of Roentgenology 2008;190(5):1163–1168. [PubMed: 18430826]
- 33.
- Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Ali A, Giovagnetti M, Opocher G, Angeli A. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab 2000;85(2):637–644. [PubMed: 10690869]
- 34.
- Jing Y, Hu J, Luo R, Mao Y, Luo Z, Zhang M, Yang J, Song Y, Feng Z, Wang Z, Cheng Q, Ma L, Yang Y, Zhong L, Du Z, Wang Y, Luo T, He W, Sun Y, Lv F, Li Q, Yang S. Prevalence and Characteristics of Adrenal Tumors in an Unselected Screening Population. 2022;175(10):1383–1391. doi:.10.7326/M22-1619 [PubMed: 36095315] [CrossRef]
- 35.
- Mayer SK, Oligny LL, Deal C, Yazbeck S, Gagné IN, Blanchard H. Childhood adrenocortical tumors: Case series and reevaluation of prognosis - A 24-year experience. J Pediatr Surg 1997;32(6):911–915. [PubMed: 9200099]
- 36.
- Angeli A, Osella G, Ali A, Terzolo M. Adrenal incidentaloma: An overview of clinical and epidemiological data from the national italian study group. Horm Res Paediatr 1997;47(4–6):279–283. [PubMed: 9167965]
- 37.
- Aron D, Terzolo M, Cawood TJ. Adrenal incidentalomas. Best Pract Res Clin Endocrinol Metab 2012;26(1):69–82. [PubMed: 22305453]
- 38.
- Hsiao HP, Kirschner LS, Bourdeau I, Keil MF, Boikos SA, Verma S, Robinson-White AJ, Nesterova M, Lacroix A, Stratakis CA. Clinical and genetic heterogeneity, overlap with other tumor syndromes, and atypical glucocorticoid hormone secretion in adrenocorticotropin-independent macronodular adrenal hyperplasia compared with other adrenocortical tumors. J Clin Endocrinol Metab 2009;94(8):2930–2937. [PMC free article: PMC2730864] [PubMed: 19509103]
- 39.
- Reznik Y, Lefebvre H, Rohmer V, Charbonnel B, Tabarin A, Rodien P, Lecomte P, Bardet S, Coffin C, Mahoudeau J. Aberrant adrenal sensitivity to multiple ligands in unilateral incidentaloma with subclinical autonomous cortisol hypersecretion: a prospective clinical study. Clin Endocrinol (Oxf) 2004;61(3):311–319. [PubMed: 15355446]
- 40.
- Lenders JWM, Duh Q-Y, Eisenhofer G, Gimenez-Roqueplo A-P, Grebe SKG, Murad MH, Naruse M, Pacak K, Young WF. Pheochromocytoma and Paraganglioma: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2014;99(6):1915–1942. [PubMed: 24893135]
- 41.
- Kopetschke R, Slisko M, Kilisli A, Tuschy U, Wallaschofski H, Fassnacht M, Ventz M, Beuschlein F, Reincke M, Reisch N, Quinkler M. Frequent incidental discovery of phaeochromocytoma: Data from a German cohort of 201 phaeochromocytoma. Eur J Endocrinol 2009;161(2):355–361. [PubMed: 19497985]
- 42.
- Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2016;101(5):1889–1916. [PubMed: 26934393]
- 43.
- Piaditis G, Markou A, Papanastasiou L, Androulakis II, Kaltsas G. Progress in aldosteronism: A review of the prevalence of primary aldosteronism in pre-hypertension and hypertension. Eur J Endocrinol 2015;172(5):R191–R203. [PubMed: 25538205]
- 44.
- Médeau V, Moreau F, Trinquart L, Clemessy M, Wémeau JL, Vantyghem MC, Plouin PF, Reznik Y. Clinical and biochemical characteristics of normotensive patients with primary aldosteronism: A comparison with hypertensive cases. Clin Endocrinol (Oxf) 2008;69(1):20–28. [PubMed: 18284637]
- 45.
- Piaditis GP, Kaltsas GA, Androulakis II, Gouli A, Makras P, Papadogias D, Dimitriou K, Ragkou D, Markou A, Vamvakidis K, Zografos G, Chrousos G. High prevalence of autonomous cortisol and aldosterone secretion from adrenal adenomas. Clin Endocrinol (Oxf) 2009;71(6):772–778. [PubMed: 19226269]
- 46.
- Sherlock M, Scarsbrook A, Abbas A, Fraser S, Limumpornpetch P, Dineen R, Stewart PM. Adrenal Incidentaloma. Endocr Rev 2020;41(6). doi:.10.1210/ENDREV/BNAA008 [PMC free article: PMC7431180] [PubMed: 32266384] [CrossRef]
- 47.
- Liao Z, Gao Y, Zhao Y, Wang Z, Wang X, Zhou J, Zhang Y. Pure androgen-secreting adrenal tumor (PASAT): A rare case report of bilateral PASATs and a systematic review. Front Endocrinol (Lausanne) 2023;14:678. [PMC free article: PMC10075358] [PubMed: 37033242]
- 48.
- Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: Update in diagnosis and management. Endocr Rev 2004;25(2):309–340. [PubMed: 15082524]
- 49.
- Zeiger MA, Siegelman SS, Hamrahian AH. Medical and surgical evaluation and treatment of adrenal incidentalomas. Journal of Clinical Endocrinology and Metabolism 2011;96(7):2004–2015. [PubMed: 21632813]
- 50.
- Fallo F, Barzon L, Boscaro M, Sonino N. Coexistence of aldosteronoma and contralateral nonfunctioning adrenal adenoma in primary aldosteronism. Am J Hypertens 1997;10(4 I):476–478. [PubMed: 9128217]
- 51.
- Satoh F, Murakami O, Takahashi K, Ueno J, Nishikawa T, Abe K, Mouri T, Sasano H. Double adenomas with different pathological and hormonal features in the left adrenal gland of a patient with Cushing’s syndrome. Clin Endocrinol (Oxf) 1997;46(2):227–234. [PubMed: 9135707]
- 52.
- Morimoto S, Sasaki S, Moriguchi J, Miki S, Kawa T, Nakamura K, Fujita H, Itoh H, Nakata T, Takeda K, Nakagawa M. Unique association of pheochromocytoma with contralateral nonfunctioning adrenal cortical adenoma. Am J Hypertens 1998;11(1):117–121. [PubMed: 9504459]
- 53.
- Chortis V, May CJH, Skordilis K, Ayuk J, Arlt W, Crowley RK. Double trouble: Two cases of dual adrenal pathologies in one adrenal mass. Endocrinol Diabetes Metab Case Rep 2019;2019(1). doi:.10.1530/EDM-18-0151 [PMC free article: PMC6432979] [PubMed: 30909165] [CrossRef]
- 54.
- Dobbie JW. Adrenocortical nodular hyperplasia: The ageing adrenal. J Pathol 1969;99(1):1–18. [PubMed: 5359219]
- 55.
- Beuschlein F, Reincke M, Allolio B, Karl M, Travis WD, Jaursch-Hancke C, Abdelhamid S, Chrousos GP. Clonal Composition of Human Adrenocortical Neoplasms. Cancer Res 1994;54(18):4927–4932. [PubMed: 7915195]
- 56.
- Gicquel C, Leblond-Francillard M, Bertagna X, Louvel A, Chapuis Y, Luton JP, Girard F, Le Bouc Y. Clonal analysis of human adrenocortical carcinomas and secreting adenomas. Clin Endocrinol (Oxf) 1994;40(4):465–477. [PubMed: 7910530]
- 57.
- Pillion DJ, Arnold P, Yang M, Stockard CR, Grizzle WE. Receptors for insulin and insulin-like growth factor-I in the human adrenal gland. Biochem Biophys Res Commun 1989;165(1):204–211. [PubMed: 2556134]
- 58.
- Reincke M, Faßnacht M, Väth S, Mora P, Allolio B. Adrenal incidentalomas: A manifestation of the metabolic syndrome? In: Endocrine Research.Vol 22.; 1996:757–761. [PubMed: 8969938]
- 59.
- Angeli A, Terzolo M. Editorial: Adrenal incidentaloma - A modern disease with old complications. Journal of Clinical Endocrinology and Metabolism 2002;87(11):4869–4871. [PubMed: 12414840]
- 60.
- Vassiliadi DA, Tzanela M, Tsatlidis V, Margelou E, Tampourlou M, Mazarakis N, Piaditis G, Tsagarakis S. Abnormal responsiveness to dexamethasone-suppressed CRH test in patients with bilateral adrenal incidentalomas. Journal of Clinical Endocrinology and Metabolism 2015;100(9):3478–3485. [PubMed: 26147608]
- 61.
- Bertagna X. Genetics of adrenal diseases in 2014: Genetics improves understanding of adrenocortical tumours. Nat Rev Endocrinol 2014;11(2):77–78. [PubMed: 25511311]
- 62.
- Lerario AM, Moraitis A, Hammer GD. Genetics and epigenetics of adrenocortical tumors. Mol Cell Endocrinol 2014;386(1–2):67–84. [PMC free article: PMC3943605] [PubMed: 24220673]
- 63.
- Bonnet-Serrano F, Bertherat J. Genetics of tumors of the adrenal cortex. Endocr Relat Cancer 2018;25(3):R131–R152. [PubMed: 29233839]
- 64.
- Zeiger M, Thompson G, Duh Q-Y, Hamrahian A, Angelos P, Elaraj D, Fishman E, Kharlip J. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons Medical Guidelines for the Management of Adrenal Incidentalomas. Endocrine Practice 2009;15(Supplement 1):1–20. [PubMed: 19632967]
- 65.
- Lau J, Balk E, Rothberg M, Ioannidis JP, DeVine D, Chew P, Kupelnick B, Miller K. Management of clinically inapparent adrenal mass. Evid Rep Technol Assess (Summ) 2002;(56):1–5. [PMC free article: PMC4781357] [PubMed: 12945556]
- 66.
- Fassnacht M, Tsagarakis S, Terzolo M, Tabarin A, Sahdev A, Newell-Price J, Pelsma I, Marina L, Lorenz K, Bancos I, Arlt W, Dekkers OM. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 2023;189(1). doi:.10.1093/EJENDO/LVAD066 [PubMed: 37318239] [CrossRef]
- 67.
- Pantalone KM, Gopan T, Remer EM, Faiman C, Ioachimescu AG, Levin HS, Siperstein A, Berber E, Shepardson LB, Bravo EL, Hamrahian AH. Change in adrenal mass size as a predictor of a malignant tumor. Endocr Pract 2010;16(4):577–587. [PubMed: 20150023]
- 68.
- Kaltsas G, Chrisoulidou A, Piaditis G, Kassi E, Chrousos G. Current status and controversies in adrenal incidentalomas. Trends in Endocrinology and Metabolism 2012;23(12):602–609. [PubMed: 23041413]
- 69.
- Blake MA, Holalkere NS, Boland GW. Imaging Techniques for Adrenal Lesion Characterization. Radiol Clin North Am 2008;46(1):65–78. [PubMed: 18328880]
- 70.
- Dinnes J, Bancos I, di Ruffano LF, Chortis V, Davenport C, Bayliss S, Sahdev A, Guest P, Fassnacht M, Deeks JJ, Arlt W. MANAGEMENT OF ENDOCRINE DISEASE: Imaging for the diagnosis of malignancy in incidentally discovered adrenal masses: a systematic review and meta-analysis. Eur J Endocrinol 2016;175(2):R51–R64. [PMC free article: PMC5065077] [PubMed: 27257145]
- 71.
- Hamrahian AH, Ioachimescu AG, Remer EM, Motta-Ramirez G, Bogabathina H, Levin HS, Reddy S, Gill IS, Siperstein A, Bravo EL. Clinical utility of noncontrast computed tomography attenuation value (hounsfield units) to differentiate adrenal adenomas/hyperplasias from nonadenomas: Cleveland clinic experience. Journal of Clinical Endocrinology and Metabolism 2005;90(2):871–877. [PubMed: 15572420]
- 72.
- Bancos I, Taylor AE, Chortis V, Sitch AJ, Jenkinson C, Davidge-Pitts CJ, Lang K, Tsagarakis S, Macech M, Riester A, Deutschbein T, Pupovac ID, Kienitz T, Prete A, Papathomas TG, Gilligan LC, Bancos C, Reimondo G, Haissaguerre M, Marina L, Grytaas MA, Sajwani A, Langton K, Ivison HE, Shackleton CHL, Erickson D, Asia M, Palimeri S, Kondracka A, Spyroglou A, Ronchi CL, Simunov B, Delivanis DA, Sutcliffe RP, Tsirou I, Bednarczuk T, Reincke M, Burger-Stritt S, Feelders RA, Canu L, Haak HR, Eisenhofer G, Dennedy MC, Ueland GA, Ivovic M, Tabarin A, Terzolo M, Quinkler M, Kastelan D, Fassnacht M, Beuschlein F, Ambroziak U, Vassiliadi DA, O’Reilly MW, Young WF, Biehl M, Deeks JJ, Arlt W, Glöckner S, Sinnott RO, Stell A, Fragoso MC, Pupovac ID, Cazenave S, Bertherat J, Libé R, Brugger C, Hahner S, Kroiss M, Ronchi CL, Vassiliadi DA, Basile V, Ingargiola E, Mannelli M, Ettaieb H, Haak HR, Kerkhofs TM, Feelders RA, Hofland J, Hofland LJ, Grytaas MA, Husebye ES, Ueland GA, Zawierucha M, Paiva I, Dennedy MC, Sherlock M, Crowley RK, Jonathan R, Sitch AJ, Giligan LC, Hughes BA, Ivison HE, Manolopoulos K, O’Neil DM, O’Reilly MW, Papathomas TG, Shackleton CHL, Taylor AE, Sutcliffe RP, Guest P, Skordilis K, Chang A, Davidge-Pitts CJ, Delivanis DA, Natt N, Nippoldt TB, Thomas M, Young WF. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: a prospective test validation study. Lancet Diabetes Endocrinol 2020;8(9):773–781. [PMC free article: PMC7447976] [PubMed: 32711725]
- 73.
- Vilar L, da Conceição Freitas M, Canadas V, Albuquerque JL, Botelho CA, Egito CS, Arruda MJ, Moura e Silva L, Coelho CE, Casulari LA, Naves LA. Adrenal Incidentalomas: Diagnostic Evaluation and Long-Term Follow-Up. Endocrine Practice 2008;14(3):269–278. [PubMed: 18463032]
- 74.
- Marty M, Gaye D, Perez P, Auder C, Nunes ML, Ferriere A, Haissaguerre M, Tabarin A. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol 2018;178(5):439–446. [PubMed: 29467231]
- 75.
- Schloetelburg W, Ebert I, Petritsch B, Weng AM, Dischinger U, Kircher S, Buck AK, Bley TA, Deutschbein T, Fassnacht M. Adrenal wash-out CT: moderate diagnostic value in distinguishing benign from malignant adrenal masses. Eur J Endocrinol 2022;186(2):183–193. [PMC free article: PMC8679842] [PubMed: 34813495]
- 76.
- Hong AR, Kim JH, Park KS, Kim KY, Lee JH, Kong SH, Lee SY, Shin CS, Kim SW, Kim SY. Optimal follow-up strategies for adrenal incidentalomas: reappraisal of the 2016 ESE-ENSAT guidelines in real clinical practice. Eur J Endocrinol 2017;177(6):475–483. [PubMed: 28870984]
- 77.
- Korobkin M, Brodeur FJ, Francis IR, Quint LE, Dunnick NR, Londy F. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. American Journal of Roentgenology 1998;170(3):747–752. [PubMed: 9490968]
- 78.
- Caoili EM, Korobkin M, Francis IR, Cohan RH, Platt JF, Dunnick NR, Raghupathi KI. Adrenal masses: Characterization with combined unenhanced delayed enhanced CT. Radiology 2002;222(3):629–633. [PubMed: 11867777]
- 79.
- Motta-Ramirez GA, Remer EM, Herts BR, Gill IS, Hamrahian AH. Comparison of CT findings in symptomatic and incidentally discovered pheochromocytomas. American Journal of Roentgenology 2005;185(3):684–688. [PubMed: 16120918]
- 80.
- Corwin MT, Badawy M, Caoili EM, Carney BW, Colak C, Elsayes KM, Gerson R, Klimkowski SP, McPhedran R, Pandya A, Pouw ME, Schieda N, Song JH, Remer EM. Incidental Adrenal Nodules in Patients Without Known Malignancy: Prevalence of Malignancy and Utility of Washout CT for Characterization-A Multiinstitutional Study. American Journal of Roentgenology 2022;219(5):804–813. [PubMed: 35731098]
- 81.
- Chambre C, McMurray E, Baudry C, Lataud M, Guignat L, Gaujoux S, Lahlou N, Guibourdenche J, Tissier F, Sibony M, Dousset B, Bertagna X, Bertherat J, Legmann P, Groussin L. The 10 Hounsfield units unenhanced computed tomography attenuation threshold does not apply to cortisol secreting adrenocortical adenomas.; 2015:325–332. [PubMed: 26243637]
- 82.
- Blake MA, Kalra MK, Sweeney AT, Lucey BC, Maher MM, Sahani D V, Halpern EF, Mueller PR, Hahn PF, Boland GW. Distinguishing benign from malignant adrenal masses: Multi-detector row CT protocol with 10-minute delay. Radiology 2006;238(2):578–585. [PubMed: 16371582]
- 83.
- Wickramarachchi BN, Meyer-Rochow GY, McAnulty K, Conaglen J V., Elston MS. Adherence to adrenal incidentaloma guidelines is influenced by radiology report recommendations. ANZ J Surg 2016;86(6):483–486. [PubMed: 25060597]
- 84.
- de Haan RR, Schreuder MJ, Pons E, Visser JJ. Adrenal Incidentaloma and Adherence to International Guidelines for Workup Based on a Retrospective Review of the Type of Language Used in the Radiology Report. Journal of the American College of Radiology 2019;16(1):50–55. [PubMed: 30253931]
- 85.
- Watari J, Vekaria S, Lin Y, Patel M, Kim H, Kang F, Lubitz S, Beninato T, Laird AM. Radiology report language positively influences adrenal incidentaloma guideline adherence. Am J Surg 2022;223(2):231–236. [PubMed: 34243951]
- 86.
- Korobkin M, Francis IR, Kloos RT, Dunnick NR. The incidental adrenal mass. Radiol Clin North Am 1996;34(5):1037–54. [PubMed: 8784395]
- 87.
- Boland GWL. Adrenal imaging: Why, when, what, and how? Part 3. The algorithmic approach to definitive characterization of the adrenal incidentaloma. American Journal of Roentgenology 2011;196(2):W109–W111. [PubMed: 21257849]
- 88.
- McDermott S, O’Connor OJ, Cronin CG, Blake MA. Radiological evaluation of adrenal incidentalomas - Current methods and future prospects. Best Pract Res Clin Endocrinol Metab 2012;26(1):21–33. [PubMed: 22305450]
- 89.
- Elsayes KM, Menias CO, Siegel CL, Narra VR, Kanaan Y, Hussain HK. Magnetic resonance characterization of pheochromocytomas in the abdomen and pelvis: Imaging findings in 18 surgically proven cases. J Comput Assist Tomogr 2010;34(4):548–553. [PubMed: 20657223]
- 90.
- Nunes ML, Rault A, Teynie J, Valli N, Guyot M, Gaye D, Belleannee G, Tabarin A. 18F-FDG PET for the identification of adrenocortical carcinomas among indeterminate adrenal tumors at computed tomography scanning. World J Surg 2010;34(7):1506–1510. [PubMed: 20396886]
- 91.
- Tessonnier L, Sebag F, Palazzo FF, Colavolpe C, De Micco C, Mancini J, Conte-Devolx B, Henry JF, Mundler O, Taïeb D. Does 18F-FDG PET/CT add diagnostic accuracy in incidentally identified non-secreting adrenal tumours?; 2008:2018–2025. [PubMed: 18566816]
- 92.
- Boland GWL, Blake MA, Holalkere NS, Hahn PF. PET/CT for the characterization of adrenal masses in patients with cancer: Qualitative versus quantitative accuracy in 150 consecutive patients. American Journal of Roentgenology 2009;192(4):956–962. [PubMed: 19304700]
- 93.
- Groussin L, Bonardel G, Silvéra S, Tissier F, Coste J, Abiven G, Libé R, Bienvenu M, Alberini JL, Salenave S, Bouchard P, Bertherat J, Dousset B, Legmann P, Richard B, Foehrenbach H, Bertagna X, Tenenbaum F. 18F-Fluorodeoxyglucose positron emission tomography for the diagnosis of adrenocortical tumors: A prospective study in 77 operated patients. Journal of Clinical Endocrinology and Metabolism 2009;94(5):1713–1722. [PubMed: 19190108]
- 94.
- Sharma P, Singh H, Dhull VVS, Suman KC S, Kumar A, Bal C, Kumar R. Adrenal Masses of Varied Etiology: Anatomical and Molecular Imaging Features on PET-CT. Clinical nuclear … 2014;00(00):1–10. [PubMed: 24217551]
- 95.
- Havekes B, King K, Lai EW, Romijn JA, Corssmit EPM, Pacak K. New imaging approaches to phaeochromocytomas and paragangliomas. Clin Endocrinol (Oxf) 2010;72(2):137–145. [PMC free article: PMC2966973] [PubMed: 19508681]
- 96.
- Gross MD, Shapiro B, Francis IR, Glazer GM, Bree RL, Arcomano MA, Schteingart DE, McLeod MK, Sanfield JA, Thompson NW, Falke THM, Sandler MP. Scintigraphic evaluation of clinically silent adrenal masses. Journal of Nuclear Medicine 1994;35(7):1145–1154. [PubMed: 8014672]
- 97.
- Hahner S, Stuermer A, Kreissl M, Reiners C, Fassnacht M, Haenscheid H, Beuschlein F, Zink M, Lang K, Allolio B, Schirbel A. [123I]Iodometomidate for molecular imaging of adrenocortical cytochrome P450 family 11B enzymes. Journal of Clinical Endocrinology and Metabolism 2008;93(6):2358–2365. [PubMed: 18397978]
- 98.
- Nieman LK, Biller BMK, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM, Edwards H. The diagnosis of Cushing’s syndrome: An endocrine society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2008;93(5):1526–1540. [PMC free article: PMC2386281] [PubMed: 18334580]
- 99.
- Morelli V, Eller-Vainicher C, Salcuni AS, Coletti F, Iorio L, Muscogiuri G, Della Casa S, Arosio M, Ambrosi B, Beck-Peccoz P, Chiodini I. Risk of new vertebral fractures in patients with adrenal incidentaloma with and without subclinical hypercortisolism: A multicenter longitudinal study. John Wiley & Sons, Ltd; 2011:1816–1821. [PubMed: 21472775]
- 100.
- Olsen H, Kjellbom A, Löndahl M, Lindgren O. Suppressed ACTH Is Frequently Unrelated to Autonomous Cortisol Secretion in Patients With Adrenal Incidentalomas. J Clin Endocrinol Metab 2019;104(2):506–512. [PubMed: 30265354]
- 101.
- Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, Tabarin A, Terzolo M, Tsagarakis S, Dekkers OM. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 2016;175(2):G1–G34. [PubMed: 27390021]
- 102.
- Tabarin A, Bardet S, Bertherat J, Dupas B, Chabre O, Hamoir E, Laurent F, Tenenbaum F, Cazalda M, Lefebvre H, Valli N, Rohmer V. Exploration and management of adrenal incidentalomas. French Society of Endocrinology Consensus. Ann Endocrinol (Paris) 2008;69(6):487–500. [PubMed: 19022420]
- 103.
- Morelli V, Masserini B, Salcuni AS, Eller-Vainicher C, Savoca C, Viti R, Coletti F, Guglielmi G, Battista C, Iorio L, Beck-Peccoz P, Ambrosi B, Arosio M, Scillitani A, Chiodini I. Subclinical hypercortisolism: Correlation between biochemical diagnostic criteria and clinical aspects. Clin Endocrinol (Oxf) 2010;73(2):161–166. [PubMed: 20184600]
- 104.
- Barzon L, Scaroni C, Sonino N, Fallo F, Paoletta A, Boscaro M. Risk Factors and Long-Term Follow-Up of Adrenal Incidentalomas1. J Clin Endocrinol Metab 1999;84(2):520–526. [PubMed: 10022410]
- 105.
- Eller-Vainicher C, Morelli V, Salcuni AS, Torlontano M, Coletti F, Iorio L, Cuttitta A, Ambrosio A, Vicentini L, Carnevale V, Beck-Peccoz P, Arosio M, Ambrosi B, Scillitani A, Chiodini I. Post-surgical hypocortisolism after removal of an adrenal incidentaloma: Is it predictable by an accurate endocrinological work-up before surgery? Eur J Endocrinol 2010;162(1):91–99. [PubMed: 19797503]
- 106.
- Eller-Vainicher C, Morelli V, Salcuni AS, Battista C, Torlontano M, Coletti F, Iorio L, Cairoli E, Beck-Peccoz P, Arosio M, Ambrosi B, Scillitani A, Chiodini I. Accuracy of several parameters of hypothalamic-pituitary-adrenal axis activity in predicting before surgery the metabolic effects of the removal of an adrenal incidentaloma. Eur J Endocrinol 2010;163(6):925–935. [PubMed: 20881060]
- 107.
- Chiodini I, Morelli V, Salcuni AS, Eller-Vainicher C, Torlontano M, Coletti F, Iorio L, Cuttitta A, Ambrosio A, Vicentini L, Pellegrini F, Copetti M, Beck-Peccoz P, Arosio M, Ambrosi B, Trischitta V, Scillitani A. Beneficial metabolic effects of prompt surgical treatment in patients with an adrenal incidentaloma causing biochemical hypercortisolism. Journal of Clinical Endocrinology and Metabolism 2010;95(6):2736–2745. [PubMed: 20375210]
- 108.
- Di Dalmazi G, Vicennati V, Garelli S, Casadio E, Rinaldi E, Giampalma E, Mosconi C, Golfieri R, Paccapelo A, Pagotto U, Pasquali R. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: A 15-year retrospective study. Lancet Diabetes Endocrinol 2014;2(5):396–405. [PubMed: 24795253]
- 109.
- Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. Journal of Clinical Endocrinology and Metabolism 2014;99(12):4462–4470. [PMC free article: PMC4255126] [PubMed: 25238207]
- 110.
- Tsagarakis S, Vassiliadi D, Thalassinos N. Endogenous subclinical hypercortisolism: Diagnostic uncertainties and clinical implications. J Endocrinol Invest 2006;29(5):471–482. [PubMed: 16794373]
- 111.
- Theodoraki A, Khoo B, Hamda A, Schwappach A, Perera S, Vanderpump MPJ, Bouloux PM. Outcomes in 125 individuals with adrenal incidentalomas from a single centre. A retrospective assessment of the 1mg overnight and low dose dexamethasone suppression tests. Hormone and Metabolic Research 2011;43(13):962–969. [PubMed: 22048862]
- 112.
- Tsagarakis S, Roboti C, Kokkoris P, Vasiliou V, Alevizaki C, Thalassinos N. Elevated post-dexamethasone suppression cortisol concentrations correlate with hormonal alterations of the hypothalamo-pituitary adrenal axis in patients with adrenal incidentalomas. Clin Endocrinol (Oxf) 1998;49(2):165–171. [PubMed: 9828902]
- 113.
- Lopez AG, Fraissinet F, Lefebvre H, Brunel V, Ziegler F. Pharmacological and analytical interference in hormone assays for diagnosis of adrenal incidentaloma. Ann Endocrinol (Paris) 2019;80(4):250–258. [PubMed: 31445667]
- 114.
- Liu MS, Lou Y, Chen H, Wang YJ, Zhang ZW, Li P, Zhu DL. Performance of DHEAS as a Screening Test for Autonomous Cortisol Secretion in Adrenal Incidentalomas: A Prospective Study. J Clin Endocrinol Metab 2022;107(5):e1789–e1796. [PubMed: 35137142]
- 115.
- Dennedy MC, Annamalai AK, Prankerd-Smith O, Freeman N, Vengopal K, Graggaber J, Koulouri O, Powlson AS, Shaw A, Halsall DJ, Gurnell M. Low DHEAS: A Sensitive and Specific Test for the Detection of Subclinical Hypercortisolism in Adrenal Incidentalomas. J Clin Endocrinol Metab 2017;102(3):786–792. [PubMed: 27797672]
- 116.
- Carafone LE, Zhang CD, Li D, Lazik N, Hamidi O, Hurtado MD, Young WF, Thomas MA, Dy BM, Lyden ML, Foster TR, McKenzie TJ, Bancos I. Diagnostic Accuracy of Dehydroepiandrosterone Sulfate and Corticotropin in Autonomous Cortisol Secretion. Biomedicines 2021;9(7). doi:.10.3390/BIOMEDICINES9070741 [PMC free article: PMC8301396] [PubMed: 34203283] [CrossRef]
- 117.
- Hána V, Ježková J, Kosák M, Kršek M, Hána V, Hill M. Novel GC-MS/MS Technique Reveals a Complex Steroid Fingerprint of Subclinical Hypercortisolism in Adrenal Incidentalomas. J Clin Endocrinol Metab 2019;104(8):3545–3556. [PubMed: 30896752]
- 118.
- Araujo-Castro M, Casals G, Hanzu FA, Pascual-Corrales E, García Cano AM, Lanza VF, Luis del Rey Mejías Á, Marchan M, Escobar-Morreale HF, Valderrabano P. Characterisation of the urinary steroid profile of patients with nonfunctioning adrenal incidentalomas: A matched controlled cross-sectional study. Clin Endocrinol (Oxf) 2023;98(2):165–176. [PubMed: 35973974]
- 119.
- Berke K, Constantinescu G, Masjkur J, Kimpel O, Dischinger U, Peitzsch M, Kwapiszewska A, Dobrowolski P, Nölting S, Reincke M, Beuschlein F, Bornstein SR, Prejbisz A, Lenders JWM, Fassnacht M, Eisenhofer G. Plasma Steroid Profiling in Patients with Adrenal Incidentaloma. Journal of Clinical Endocrinology and Metabolism 2022;107(3):E1181–E1192. [PubMed: 34665854]
- 120.
- Grozinsky-Glasberg S, Szalat A, Benbassat CA, Gorshtein A, Weinstein R, Hirsch D, Shraga-Slutzky I, Tsvetov G, Gross DJ, Shimon I. Clinically silent chromaffin-cell tumors: Tumor characteristics and long-term prognosis in patients with incidentally discovered pheochromocytomas. J Endocrinol Invest 2010;33(10):739–744. [PubMed: 20479567]
- 121.
- Lenders JWM, Pacak K, Walther MM, Marston Linehan W, Mannelli M, Friberg P, Keiser HR, Goldstein DS, Eisenhofer G. Biochemical diagnosis of pheochromocytoma: Which test is best? J Am Med Assoc 2002;287(11):1427–1434. [PubMed: 11903030]
- 122.
- Eisenhofer G, Prejbisz A, Peitzsch M, Pamporaki C, Masjkur J, Rogowski-Lehmann N, Langton K, Tsourdi E, Peczkowska M, Fliedner S, Deutschbein T, Megerle F, Timmers HJLM, Sinnott R, Beuschlein F, Fassnacht M, Januszewicz A, Lenders JWM. Biochemical Diagnosis of Chromaffin Cell Tumors in Patients at High and Low Risk of Disease: Plasma versus Urinary Free or Deconjugated O-Methylated Catecholamine Metabolites. Clin Chem 2018;64(11):1646–1656. [PubMed: 30097498]
- 123.
- Sane T, Schalin-Jäntti C, Raade M. Is biochemical screening for pheochromocytoma in adrenal incidentalomas expressing low unenhanced attenuation on computed tomography necessary? Journal of Clinical Endocrinology and Metabolism 2012;97(6):2077–2083. [PubMed: 22492870]
- 124.
- Canu L, Van Hemert JAW, Kerstens MN, Hartman RP, Khanna A, Kraljevic I, Kastelan D, Badiu C, Ambroziak U, Tabarin A, Haissaguerre M, Buitenwerf E, Visser A, Mannelli M, Arlt W, Chortis V, Bourdeau I, Gagnon N, Buchy M, Borson-Chazot F, Deutschbein T, Fassnacht M, Hubalewska-Dydejczyk A, Motyka M, Rzepka E, Casey RT, Challis BG, Quinkler M, Vroonen L, Spyroglou A, Beuschlein F, Lamas C, Young WF, Bancos I, Timmers HJLM. CT Characteristics of Pheochromocytoma: Relevance for the Evaluation of Adrenal Incidentaloma. Journal of Clinical Endocrinology and Metabolism 2018;104(2):312–318. [PubMed: 30383267]
- 125.
- Haissaguerre M, Courel M, Caron P, Denost S, Dubessy C, Gosse P, Appavoupoulle V, Belleannée G, Jullié ML, Montero-Hadjadje M, Yon L, Corcuff JB, Fagour C, Mazerolles C, Wagner T, Nunes ML, Anouar Y, Tabarin A. Normotensive incidentally discovered pheochromocytomas display specific biochemical, cellular, and molecular characteristics. Journal of Clinical Endocrinology and Metabolism 2013;98(11):4346–4354. [PubMed: 24001749]
- 126.
- Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ, Hammer GD. Adrenocortical carcinoma. Endocr Rev 2014;35(2):282–326. [PMC free article: PMC3963263] [PubMed: 24423978]
- 127.
- Phornphutkul C, Okubo T, Wu K, Harel Z, Tracy TF, Pinar H, Chen S, Gruppuso PA, Goodwin G. Aromatase P450 expression in a feminizing adrenal adenoma presenting as isosexual precocious puberty. Journal of Clinical Endocrinology and Metabolism 2001;86(2):649–652. [PubMed: 11158024]
- 128.
- Goto T, Murakami O, Sato F, Haraguchi M, Yokoyama K, Sasano H. Oestrogen producing adrenocortical adenoma: Clinical, biochemical and immunohistochemical studies. Clin Endocrinol (Oxf) 1996;45(5):643–648. [PubMed: 8977764]
- 129.
- Fukushima A, Okada Y, Tanikawa T, Kawahara C, Misawa H, Kanda K, Morita E, Sasano H, Tanaka Y. Virilizing adrenocortical adenoma with Cushing’s syndrome, thyroid papillary carcinoma and hypergastrinemia in a middle-aged woman. Endocr J 2003;50(2):179–187. [PubMed: 12803238]
- 130.
- Rodríguez-Gutiérrez R, Bautista-Medina MA, Teniente-Sanchez AE, Zapata-Rivera MA, Montes-Villarreal J. Pure Androgen-Secreting Adrenal Adenoma Associated with Resistant Hypertension. Case Rep Endocrinol 2013;2013:1–4. [PMC free article: PMC3681270] [PubMed: 23819074]
- 131.
- Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet 2014;383(9935):2152–67. [PubMed: 24503135]
- 132.
- Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA, Torpy DJ. Diagnosis and treatment of primary adrenal insufficiency: An endocrine society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2016;101(2):364–389. [PMC free article: PMC4880116] [PubMed: 26760044]
- 133.
- Quayle FJ, Spitler JA, Pierce RA, Lairmore TC, Moley JF, Brunt LM. Needle biopsy of incidentally discovered adrenal masses is rarely informative and potentially hazardous. Surgery 2007;142(4):497–504. [PubMed: 17950341]
- 134.
- Bancos I, Tamhane S, Shah M, Delivanis DA, Alahdab F, Arlt W, Fassnacht M, Murad MH. Diagnosis of endocrine disease: The diagnostic performance of adrenal biopsy: A systematic review and meta-analysis. Eur J Endocrinol 2016;175(2):R65–R80. [PubMed: 27257146]
- 135.
- Mazzaglia PJ, Monchik JM. Limited value of adrenal biopsy in the evaluation of adrenal neoplasm: a decade of experience. Arch Surg 2009;144(5):465–470. [PubMed: 19451490]
- 136.
- Lumachi F, Borsato S, Tregnaghi A, Marino F, Fassina A, Zucchetta P, Marzola MC, Cecchin D, Bui F, Iacobone M, Favia G. High risk of malignancy in patients with incidentally discovered adrenal masses: Accuracy of adrenal imaging and image-guided fine-needle aspiration cytology. Tumori 2007;93(3):269–274. [PubMed: 17679462]
- 137.
- Harisinghani MG, Maher MM, Hahn PF, Gervais DA, Jhaveri K, Varghese J, Mueller PR. Predictive value of benign percutaneous adrenal biopsies in oncology patients. Clin Radiol 2002;57(10):898–901. [PubMed: 12413913]
- 138.
- Welch TJ, Sheedy PF, Stephens DH, Johnson CM, Swensen SJ. Percutaneous adrenal biopsy: Review of a 10-year experience. Radiology 1994;193(2):341–344. [PubMed: 7972740]
- 139.
- Vanderveen KA, Thompson SM, Callstrom MR, Young WF, Grant CS, Farley DR, Richards ML, Thompson GB. Biopsy of pheochromocytomas and paragangliomas: Potential for disaster. Surgery 2009;146(6):1158–1166. [PubMed: 19958944]
- 140.
- Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab 2009;23(2):273–289. [PubMed: 19500769]
- 141.
- Sutton St. JM, Sheps SG, Lie JT. Prevalence of clinically unsuspected pheochromocytoma. Review of a 50-year autopsy series. Mayo Clin Proc 1981;56(6):354–360. [PubMed: 6453259]
- 142.
- Bülow B, Jansson S, Juhlin C, Steen L, Thorén M, Wahrenberg H, Valdemarsson S, Wängberg B, Ahrén B. Adrenal incidentaloma - Follow up results from a Swedish prospective study. Eur J Endocrinol 2006;154(3):419–423. [PubMed: 16498055]
- 143.
- Bernini GP, Moretti A, Oriandini C, Bardini M, Taurino C, Salvetti A. Long-term morphological and hormonal follow-up in a single unit on 115 patients with adrenal incidentalomas. Br J Cancer 2005;92(6):1104–1109. [PMC free article: PMC2361933] [PubMed: 15770213]
- 144.
- Terzolo M, Bovio S, Reimondo G, Pia A, Osella G, Borretta G, Angeli A. Subclinical Cushing’s syndrome in adrenal incidentalomas. Endocrinol Metab Clin North Am 2005;34(2):423–439. [PubMed: 15850851]
- 145.
- Belmihoub I, Silvera S, Sibony M, Dousset B, Legmann P, Bertagna X, Bertherat J, Assié G. From benign adrenal incidentaloma to adrenocortical carcinoma: An exceptional random event. Eur J Endocrinol 2017;176(6):K15–K19. [PubMed: 28348073]
- 146.
- Rebielak ME, Wolf MR, Jordan R, Oxenberg JC. Adrenocortical carcinoma arising from an adrenal adenoma in a young adult female. J Surg Case Rep 2019;2019(7):rjz200. [PMC free article: PMC6622115] [PubMed: 31308928]
- 147.
- Ronchi CL, Sbiera S, Leich E, Henzel K, Rosenwald A, Allolio B, Fassnacht M. Single Nucleotide Polymorphism Array Profiling of Adrenocortical Tumors - Evidence for an Adenoma Carcinoma Sequence? Veitia RA, ed. PLoS One 2013;8(9):e73959. [PMC free article: PMC3774745] [PubMed: 24066089]
- 148.
- Assié G, Letouzé E, Fassnacht M, Jouinot A, Luscap W, Barreau O, Omeiri H, Rodriguez S, Perlemoine K, René-Corail F, Elarouci N, Sbiera S, Kroiss M, Allolio B, Waldmann J, Quinkler M, Mannelli M, Mantero F, Papathomas T, De Krijger R, Tabarin A, Kerlan V, Baudin E, Tissier F, Dousset B, Groussin L, Amar L, Clauser E, Bertagna X, Ragazzon B, Beuschlein F, Libé R, de Reyniès A, Bertherat J. Integrated genomic characterization of adrenocortical carcinoma. Nat Genet 2014;46(6):607–612. [PubMed: 24747642]
- 149.
- Libè R, Dall’Asta C, Barbetta L, Baccarelli A, Beck-Peccoz P, Ambrosi B. Long-term follow-up study of patients with adrenal incidentalomas. Eur J Endocrinol 2002;147(4):489–494. [PubMed: 12370111]
- 150.
- Androulakis II, Kaltsas G, Piaditis G, Grossman AB. The clinical significance of adrenal incidentalomas. Eur J Clin Invest 2011;41(5):552–560. [PubMed: 21210792]
- 151.
- Terzolo M, Pia A, Alì A, Osella G, Reimondo G, Bovio S, Daffara F, Procopio M, Paccotti P, Borretta G, Angeli A. Adrenal incidentaloma: A new cause of the metabolic syndrome? Journal of Clinical Endocrinology and Metabolism 2002;87(3):998–1003. [PubMed: 11889151]
- 152.
- Morelli V, Reimondo G, Giordano R, Della Casa S, Policola C, Palmieri S, Salcuni AS, Dolci A, Mendola M, Arosio M, Ambrosi B, Scillitani A, Ghigo E, Beck-Peccoz P, Terzolo M, Chiodini I. Long-term follow-up in adrenal incidentalomas: An Italian multicenter study. Journal of Clinical Endocrinology and Metabolism 2014;99(3):827–834. [PubMed: 24423350]
- 153.
- Deutschbein T, Reimondo G, Di Dalmazi G, Bancos I, Patrova J, Vassiliadi DA, Nekić AB, Debono M, Lardo P, Ceccato F, Petramala L, Prete A, Chiodini I, Ivović M, Pazaitou-Panayiotou K, Alexandraki KI, Hanzu FA, Loli P, Yener S, Langton K, Spyroglou A, Kocjan T, Zacharieva S, Valdés N, Ambroziak U, Suzuki M, Detomas M, Puglisi S, Tucci L, Delivanis DA, Margaritopoulos D, Dusek T, Maggio R, Scaroni C, Concistrè A, Ronchi CL, Altieri B, Mosconi C, Diamantopoulos A, Iñiguez-Ariza NM, Vicennati V, Pia A, Kroiss M, Kaltsas G, Chrisoulidou A, Marina L V., Morelli V, Arlt W, Letizia C, Boscaro M, Stigliano A, Kastelan D, Tsagarakis S, Athimulam S, Pagotto U, Maeder U, Falhammar H, Newell-Price J, Terzolo M, Fassnacht M. Age-dependent and sex-dependent disparity in mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: an international, retrospective, cohort study. Lancet Diabetes Endocrinol 2022;10(7):499–508. [PMC free article: PMC9679334] [PubMed: 35533704]
- 154.
- Toniato A, Merante-Boschin I, Opocher G, Pelizzo MR, Schiavi F, Ballotta E. Surgical versus conservative management for subclinical cushing syndrome in adrenal incidentalomas: A prospective randomized study. Ann Surg 2009;249(3):388–391. [PubMed: 19247023]
- 155.
- Sereg M, Szappanos Á, Tóke J, Karlinger K, Feldman K, Kaszper É, Varga I, Gláz E, Rácz K, Tóth M. Atherosclerotic risk factors and complications in patients with non-functioning adrenal adenomas treated with or without adrenalectomy: A long-term follow-up study. Eur J Endocrinol 2009;160(4):647–655. [PubMed: 19174533]
- 156.
- Tsuiki M, Tanabe A, Takagi S, Naruse M, Takano K. Cardiovascular risks and their long-term clinical outcome in patients with subclinical Cushing’s syndrome. Endocr J 2008;55(4):737–745. [PubMed: 18506093]
- 157.
- Chiodini I, Viti R, Coletti F, Guglielmi G, Battista C, Ermetici F, Morelli V, Salcuni A, Carnevale V, Urbano F, Muscarella S, Ambrosi B, Arosio M, Beck-Peccoz P, Scillitani A. Eugonadal male patients with adrenal incidentalomas and subclinical hypercortisolism have increased rate of vertebral fractures. Clin Endocrinol (Oxf) 2009;70(2):208–213. [PubMed: 18547342]
- 158.
- Tauchmanová L, Pivonello R, De Martino MC, Rusciano A, De Leo M, Ruosi C, Mainolfi C, Lombardi G, Salvatore M, Colao A. Effects of sex steroids on bone in women with subclinical or overt endogenous hypercortisolism. Eur J Endocrinol 2007;157(3):359–366. [PubMed: 17766720]
- 159.
- Chiodini I, Mascia ML, Muscarella S, Battista C, Minisola S, Arosio M, Santini SA, Guglielmi G, Carnevale V, Scillitani A. Subclinical hypercortisolism among outpatients referred for osteoporosis. Ann Intern Med 2007;147(8):541–548. [PubMed: 17938392]
- 160.
- Chiodini I, Vainicher CE, Morelli V, Palmieri S, Cairoli E, Salcuni AS, Copetti M, Scillitani A. Endogenous subclinical hypercortisolism and bone: A clinical review. Eur J Endocrinol 2016;175(6):R265–R282. [PubMed: 27412441]
- 161.
- Guerrieri M, Campagnacci R, Patrizi A, Romiti C, Arnaldi G, Boscaro M. Primary adrenal hypercortisolism: Minimally invasive surgical treatment or medical therapy? A retrospective study with long-term follow-up evaluation. Surg Endosc 2010;24(10):2542–2546. [PubMed: 20336323]
- 162.
- Peppa M, Boutati E, Koliaki C, Papaefstathiou N, Garoflos E, Economopoulos T, Hadjidakis D, Raptis SA. Insulin resistance and metabolic syndrome in patients with nonfunctioning adrenal incidentalomas: A cause-effect relationship? Metabolism 2010;59(10):1435–1441. [PubMed: 20153874]
- 163.
- Garrapa GGM, Pantanetti P, Arnaldi G, Mantero F, Faloia E. Body Composition and Metabolic Features in Women with Adrenal Incidentaloma or Cushing’s Syndrome. J Clin Endocrinol Metab 2001;86(11):5301–5306. [PubMed: 11701696]
- 164.
- Fernández-Real JM, Ricart Engel W, Simó R, Salinas I, Webb SM. Study of glucose tolerance in consecutive patients harbouring incidental adrenal tumours. Clin Endocrinol (Oxf) 1998;49(1):53–61. [PubMed: 9797847]
- 165.
- Yener S, Genc S, Akinci B, Secil M, Demir T, Comlekci A, Ertilav S, Yesil S. Carotid intima media thickness is increased and associated with morning cortisol in subjects with non-functioning adrenal incidentaloma. Endocrine 2009;35(3):365–370. [PubMed: 19277910]
- 166.
- Yener S, Baris M, Secil M, Akinci B, Comlekci A, Yesil S. Is there an association between non-functioning adrenal adenoma and endothelial dysfunction? J Endocrinol Invest 2011;34(4):265–270. [PubMed: 20530985]
- 167.
- Ermetici F, Dall’Asta C, Malavazos AE, Coman C, Morricone L, Montericcio V, Ambrosi B. Echocardiographic alterations in patients with non-functioning adrenal incidentaloma. J Endocrinol Invest 2008;31(6):573–577. [PubMed: 18591893]
- 168.
- Androulakis II, Kaltsas GA, Kollias GE, Markou AC, Gouli AK, Thomas DA, Alexandraki KI, Papamichael CM, Hadjidakis DJ, Piaditis GP. Patients with apparently nonfunctioning adrenal incidentalomas may be at increased cardiovascular risk due to excessive cortisol secretion. Journal of Clinical Endocrinology and Metabolism 2014;99(8):2754–2762. [PubMed: 24712565]
- 169.
- Lopez D, Luque-Fernandez MA, Steele A, Adler GK, Turchin A, Vaidya A. “Nonfunctional” Adrenal Tumors and the Risk for Incident Diabetes and Cardiovascular Outcomes. Ann Intern Med 2016;165(8):533. [PMC free article: PMC5453639] [PubMed: 27479926]
- 170.
- Elhassan YS, Alahdab F, Prete A, Delivanis DA, Khanna A, Prokop L, Murad MH, O’Reilly MW, Arlt W, Bancos I. Natural History of Adrenal Incidentalomas With and Without Mild Autonomous Cortisol Excess. Ann Intern Med 2019;171(2):107. [PubMed: 31234202]
- 171.
- Terzolo M, Reimondo G. Insights on the Natural History of Adrenal Incidentalomas. Ann Intern Med 2019;171(2):135. [PubMed: 31234201]
- 172.
- Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, Hartman RP, Dean DS, Thomas MA, Shah MZ, Herndon J, McKenzie TJ, Arlt W, Young WF, Bancos I. Clinical, Biochemical, and Radiological Characteristics of a Single-Center Retrospective Cohort of 705 Large Adrenal Tumors. Mayo Clin Proc Innov Qual Outcomes 2018;2(1):30–39. [PMC free article: PMC6124341] [PubMed: 30225430]
- 173.
- Nieman LK, Biller BMK, Findling JW, Murad MH, Newell-Price J, Savage MO, Tabarin A. Treatment of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2015;100(8):2807–2831. [PMC free article: PMC4525003] [PubMed: 26222757]
- 174.
- Nieman LK. Approach to the patient with an adrenal incidentaloma. Journal of Clinical Endocrinology and Metabolism 2010;95(9):4106–4113. [PMC free article: PMC2936073] [PubMed: 20823463]
- 175.
- Terzolo M, Bovio S, Pia A, Reimondo G, Angeli A. Management of adrenal incidentaloma. Best Pract Res Clin Endocrinol Metab 2009;23(2):233–243. [PubMed: 19500766]
- 176.
- Calissendorff J, Juhlin CC, Sundin A, Bancos I, Falhammar H. Adrenal myelolipomas. Lancet Diabetes Endocrinol 2021;9(11):767–775. [PMC free article: PMC8851410] [PubMed: 34450092]
- 177.
- Di Dalmazi G, Berr CM, Fassnacht M, Beuschlein F, Reincke M. Adrenal Function After Adrenalectomy for Subclinical Hypercortisolism and Cushing’s Syndrome: A Systematic Review of the Literature. J Clin Endocrinol Metab 2014;99(8):2637–2645. [PubMed: 24878052]
- 178.
- Khawandanah D, ElAsmar N, Arafah BM. Alterations in hypothalamic-pituitary-adrenal function immediately after resection of adrenal adenomas in patients with Cushing’s syndrome and others with incidentalomas and subclinical hypercortisolism. Endocrine 2019;63(1):140–148. [PubMed: 30259310]
- 179.
- Kastelan D, Kraljevic I, Dusek T, Knezevic N, Solak M, Gardijan B, Kralik M, Poljicanin T, Skoric-Polovina T, Kastelan Z. The clinical course of patients with adrenal incidentaloma: Is it time to reconsider the current recommendations? Eur J Endocrinol 2015;173(2):275–282. [PubMed: 26024670]
- 180.
- Yeomans H, Calissendorff J, Volpe C, Falhammar H, Mannheimer B. Limited value of long-term biochemical follow-up in patients with adrenal incidentalomas-a retrospective cohort study. BMC Endocr Disord 2015;15(1):6. [PMC free article: PMC4377053] [PubMed: 25887139]
- 181.
- Muth A, Taft C, Hammarstedt L, Björneld L, Hellström M, Wängberg B. Patient-reported impacts of a conservative management programme for the clinically inapparent adrenal mass.; 2013:228–236. [PMC free article: PMC3726925] [PubMed: 23250632]
