Context and Policy Issues
Inflammatory bowel disease is a chronic, disabling, and progressive inflammatory condition that includes Crohn’s disease, which affects mainly in the lower part of the small intestine and the colon, and ulcerative colitis, which affects portions of the large intestine. Both tend to be first diagnosed in young adults, can require surgical intervention, and typically involve periods of relapse and remission,1,2 The conditions affected about 270,000 Canadians in 2018, with more than 10,200 new cases diagnosed every year.3,4 Pharmacological management of Crohn’s disease and ulcerative colitis typically involves a stepwise approach, and remission induction usually includes anti-inflammatory drugs such as 5-aminosalicylate (5-ASA) for mild diseases, and corticosteroids for moderate and severe disease, or immune modifying agents such as thiopurine (azathioprine, 6-mercaptopurine), methotrexate, with addition of or switching to biologics for refractory cases.5–8 Biologics, antibodies with targeted action on inflammation proteins, that are used in the treatment of inflammatory bowel disease usually include anti-tumour necrosis factor (anti-TNF) such as adalimumab and infliximab, and non anti-TNF such as vedolizumab and ustekinumab.9 Numerous guidelines and position statements have recommended the use of biologics as a second or third line option in 5-ASA-, thiopurine-, or steroid-resistant Crohn’s disease and ulcerative colitis.10–25
Perianal fistulizing Crohn’s disease, a condition in which enterocutaneous (perianal) fistulas are developed in many patients of Crohn’s disease, is difficult to treat despite the addition of antibiotics.26 Limited evidence from a small size randomized controlled trial (RCT) has shown that infliximab is more efficacious than placebo in healing draining fistulas in patients with perianal fistulizing Crohn’s disease.27 Initiation of biologics earlier in the course of Crohn’s disease in combination with azathioprine could be more effective than conventional therapy in remission induction in another small size RCT.28
This Rapid Response report aims to review the evidence-based guidelines associated with the early (first line) use of biologics in the sequencing of pharmacological treatments for patients with moderate to severe Crohn’s disease and ulcerative colitis.
Research Question
What are the evidence-based guidelines associated with the early use of biologics in the sequencing of pharmacological treatments for patients with moderate to severe Crohn’s disease or ulcerative colitis?
Key Findings
The evidence-based guidelines from Canadian Association of Gastroenterology recommended that for patients with perianal fistulizing Crohn’s disease, anti-TNF such as infliximab or adalimumab should be used as initial treatment, possibly combined with thiopurine or methotrexate. The guideline stated that the recommendation was based on evidence of very low quality.
Methods
A limited literature search was conducted on key resources including Medline, the Cochrane Library, University of York Centre for Reviews and Dissemination (CRD) databases, Canadian and major international health technology agencies, as well as a focused Internet search. Methodological filters were applied to limit the retrieval to guidelines only. Where possible, retrieval was limited to the human population. The search was also limited to English language documents published between January 1, 2013 and December 20, 2018.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in , they were duplicate publications, or were published prior to 2013.
Critical Appraisal of Individual Studies
The included guidelines were assessed using the AGREE II checklist.29 Summary scores were not calculated for the included studies; rather, a review of the strengths and limitations of each included study were described narratively.
Summary of Evidence
Quantity of Research Available
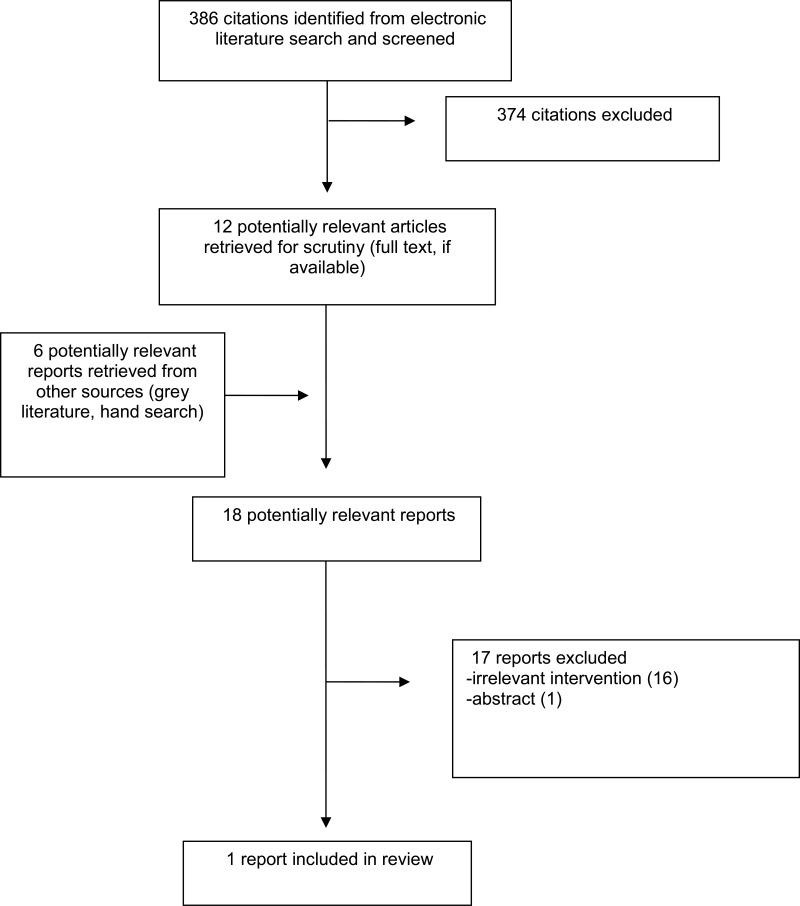
A total of 386 citations were identified in the literature search. Following screening of titles and abstracts, 374 citations were excluded and 12 potentially relevant reports from the electronic search were retrieved for full-text review. Six potentially relevant publications were retrieved from the grey literature search. Of these potentially relevant articles, 17 publications were excluded for various reasons, while one publication met the inclusion criteria and were included in this report. Appendix 1 describes the PRISMA flowchart of the study selection.
Summary of Study Characteristics
The included guideline is an evidence-based practice guideline developed in 2019 by the Canadian Association of Gastroenterology for the medical management of perianal fistulizing Crohn’s disease in patients of all ages.30 Guideline content and recommendations were based on a structured review of the literature up to April 2016. Statements were developed using the Delphi process, then finalized and voted on by a group of specialists. The evidence and recommendation ratings were adopted from the classification developed by the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) workgroup.
Additional characteristics of the included guideline are detailed in Appendix 2.
Summary of Critical Appraisal
The included guideline30 has a clear scope and purpose, the recommendations are specific and unambiguous, methods used for formulating the recommendations are clearly described, health benefits, side effects, and risks were stated in the recommendations, and the procedures for updating the guidelines provided and target users of the guideline are clearly defined. The methods for searching for and selecting the evidence were clear. Potential cost implications of applying the recommendation were not included. It was unclear whether the guideline was piloted among target users, or whether patients’ views and preferences were sought.
Details of the critical appraisal of the included studies are presented in Appendix 3.
Summary of Findings
Guidelines
The evidence-based guidelines from the Canadian Association of Gastroenterology Statement30 on the use of early biologics recommend:
“In patients with Crohn’s disease and evidence of fistulizing disease, we suggest the use of antibiotic therapy for initial management to achieve symptomatic response. GRADE: Conditional recommendation, very low-quality evidence
In patients with Crohn’s disease and evidence of fistulizing disease, we recommend the use of anti-TNF therapy, to induce symptomatic response. GRADE: Strong recommendation, very low-quality evidence.
In patients with Crohn’s disease and evidence of fistulizing disease who have achieved symptomatic response on anti-TNF therapy, we suggest the use of continued therapy, to achieve and maintain complete remission. GRADE: Conditional recommendation, low-quality evidence.
In patients with Crohn’s disease and evidence of fistulizing disease, when starting anti-TNF therapy, we suggest it be combined with a thiopurine or methotrexate over monotherapy to optimize pharmacokinetic parameters. GRADE: Conditional recommendation, low-quality evidence for infliximab, very low-quality evidence for adalimumab” (p 6)
The statements are applicable to patients of all ages with perianal fistulizing Crohn’s disease. The main findings of the included guideline are presented in Appendix 4.
Limitations
The identified guideline on the early use of biologics was limited to one subset of patients with perianal fistulising Crohn’s, based on evidence of very-low quality. The estimate of effect is therefore very uncertain. No guidelines that recommended first line of biologics for the treatment of ulcerative colitis or of luminal Crohn’s disease were identified and therefore the results may not generalize to those populations.
Conclusions and Implications for Decision or Policy Making
The evidence-based guidelines from the Canadian Association of Gastroenterology recommend that for patients with perianal fistulizing Crohn’s disease, following the use of antibiotics for infection control, anti-TNF medications such as infliximab or adalimumab should be used as initial treatment, possibly combined with thiopurine or methotrexate. This treatment should be continued for patients who show symptomatic response. These recommendations were based on evidence of very low quality.
The majority of current guidelines have recommended the use of biologics as a second or third line option in 5-ASA-, thiopurine-, or steroid-resistant Crohn’s disease and ulcerative colitis. Development of guidelines based on clinical efficacy and effectiveness of early use of biologics in the treatment of Crohn’s disease and ulcerative colitis are needed.
References
- 1.
Monstad
I, Hovde
O, Solberg
IC, A Moum
B. Clinical course and prognosis in ulcerative colitis: results from population-based and observational studies.
Ann Gastroenterol. 2014;27(2):95–104. [
PMC free article: PMC3982647] [
PubMed: 24733679]
- 2.
Baumgart
DC, Sandborn
WJ. Crohn’s disease.
Lancet. 2012;380(9853):1590–1605. [
PubMed: 22914295]
- 3.
- 4.
- 5.
Al Hashash
J, Regueiro
M. Overview of medical management of high-risk, adult patients with moderate to severe Crohn disease. In: Post
TW, ed.
UpToDate. Waltham (MA): UpToDate; 2018:
uptodate.com. Accessed 2019 Jan 28.
- 6.
Bousvaros
A, Setty
M, Kaplan
J. Management of mild to moderate ulcerative colitis in children and adolescents. In: Post
TW, ed.
UpToDate. Waltham (MA): UpToDate; 2017:
uptodate.com. Accessed 2019 Jan 28.
- 7.
Bousvaros
A. Overview of the management of Crohn disease in children and adolescents. In: Post
TW, ed.
UpToDate. Waltham (MA): UpToDate; 2018:
uptodate.com. Accessed 2019 Jan 28.
- 8.
Wisniewski
A, Danese
S, Peyrin-Biroulet
L. Evolving treatment algorithms in Crohn’s disease.
Curr Drug Targets. 2018;19(7):782–790. [
PubMed: 27280794]
- 9.
- 10.
Lichtenstein
GR, Loftus
EV, Isaacs
KL, Regueiro
MD, Gerson
LB, Sands
BE. ACG clinical guideline: management of Crohn’s disease in adults.
Am J Gastroenterol. 2018;113(4):481–517. [
PubMed: 29610508]
- 11.
Peyrin-Biroulet
L, Bouhnik
Y, Roblin
X, et al. French national consensus clinical guidelines for the management of Crohn’s disease.
Dig Liver Dis. 2017;49(4):368–377. [
PubMed: 28087156]
- 12.
Ruemmele
FM, Veres
G, Kolho
KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease.
J Crohns Colitis. 2014;8(10):1179–1207. [
PubMed: 24909831]
- 13.
Gomollon
F, Dignass
A, Annese
V, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management.
J Crohns Colitis. 2017;11(1):3–25. [
PubMed: 27660341]
- 14.
Mayberry
JF, Lobo
A, Ford
AC, Thomas
A. NICE clinical guideline (CG152): the management of Crohn’s disease in adults, children and young people.
Aliment Pharmacol Ther. 2013;37(2):195–203. [
PubMed: 23151246]
- 15.
Ueno
F, Matsui
T, Matsumoto
T, et al. Evidence-based clinical practice guidelines for Crohn’s disease, integrated with formal consensus of experts in Japan.
J Gastroenterol. 2013;48(1):31–72. [
PMC free article: PMC3541931] [
PubMed: 23090001]
- 16.
- 17.
- 18.
- 19.
Turner
D, Ruemmele
FM, Orlanski-Meyer
E, et al. Management of paediatric ulcerative colitis, part 2: acute severe colitis; an evidence-based consensus guideline from ECCO and ESPGHAN.
J Pediatr Gastroenterol Nutr. 2018;30:30. [
PubMed: 30044358]
- 20.
Turner
D, Ruemmele
FM, Orlanski-Meyer
E, et al. Management of paediatric ulcerative colitis, part 1: ambulatory care- an evidence-based guideline from ECCO and ESPGHAN.
J Pediatr Gastroenterol Nutr. 2018;30:30. [
PubMed: 30044357]
- 21.
Stenke
E, Hussey
S. Ulcerative colitis: management in adults, children and young people (NICE Clinical Guideline CG166).
Arch. 2014;99(5):194–197. [
PubMed: 24821990]
- 22.
Gomollon
F, Garcia-Lopez
S, Sicilia
B, Gisbert
JP, Hinojosa
J, Grupo Espa~nol de Trabajo en Enfermedad de Crohn y Colitis U. Therapeutic guidelines on ulcerative colitis: a GRADE methodology based effort of GETECCU.
Gastroenterol Hepatol. 2013;36(2):104–114. [
PubMed: 23332546]
- 23.
National Clinical Guideline Centre. Ulcerative colitis: management in adults, children and young people.
National Institute for Health and Clinical Excellence: guidance. London (GB): Royal College of Physicians (UK); 2013. [
PubMed: 25340222]
- 24.
- 25.
- 26.
Williams
D, Coller
J, Corman
M, Nugent
F, Veidenheimer
M. Anal complications in Crohn’s disease.
Dis Colon Rectum. 1981;24. [
PubMed: 7472097]
- 27.
Present
D, Rutgeerts
P, Targan
S, Hanauer
S, Mayer
L, van Hogezand
RA. Infliximab for the treatment of fistulas in patients with Crohn’s disease.
N Engl J Med. 1999;340. [
PubMed: 10228190]
- 28.
D’Haens
G. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial.
Lancet. 2008;371. [
PubMed: 18295023]
- 29.
- 30.
Steinhart
AH, Panaccione
R, Targownik
L, et al. Clinical practice guideline for the medical management of perianal fistulizing Crohn’s disease: the Toronto consensus.
Inflamm Bowel Dis. 2019;25(1):1–13. [
PubMed: 30099529]
Appendix 1. Selection of Included Studies
Appendix 2. Characteristics of Included Publications
Table 2Characteristics of Included Guidelines
View in own window
| Guideline Development Group, Year | Scope and Interventions | Target Population; Intended users | Evidence Collection, Selection, and Synthesis | Recommendations Development and Evaluation | Grading system |
|---|
| Canadian Association of Gastroenterology,30 Canada, Steinhart, et al. 2019 | Guideline for the medical management of perianal fistulizing Crohn’s disease Interventions: medical management for the condition | Adults and children with perianal fistulizing Crohn’s disease; Intended users assumed to be those providing multidisciplinary medical management for those with perianal fistulising Crohn’s disease | Systematic structured evidence review done by the Canadian Association of Gastroenterology (literature search up to April 2016 for MEDLINE, EMBASE, CENTRAL) | Statements were developed through an iterative online platform using a modified Delphi process, then finalized, and voted on by a group of specialists. | The evidence and recommendation rating were adopted from the classification developed by the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) workgroup. The GRADE system primarily involves consideration of the following factors: overall study quality (or overall risk of bias or study limitations), consistency of evidence, directness of evidence, and precision of evidence. |
Appendix 3. Critical Appraisal of Included Publications
Table 3Summary of Critical Appraisal of Included Guideline using AGREE II5
View in own window
| First Author, Publication Year | Strengths | Limitations |
|---|
| Canadian Association of Gastroenterology Statement, 201930, Steinhart, et al. |
scope and purpose of the guidelines are clear the recommendations are specific and unambiguous the method for searching for and selecting the evidence are clear methods used for formulating the recommendations are clearly described health benefits, side effects and risks were stated in the recommendations procedure for updating the guidelines provided target users of the guideline are clearly defined
|
unclear whether the guideline was piloted among target users unclear whether patients’ views and preferences were sought potential cost implications of applying the recommendation not included
|
Appendix 4. Main Study Findings and Author’s Conclusions
Table 4Main Study Findings and Authors’ Conclusions
View in own window
| Strength of Evidence and Recommendations | Authors’ Conclusions |
|---|
| Canadian Association of Gastroenterology (Evidence-based Guideline), 201930, Steinhart, et al. |
|---|
“3: In patients with Crohn’s disease and evidence of fistulizing disease, we suggest the use of antibiotic therapy for initial management to achieve symptomatic response. GRADE: Conditional recommendation, very low-quality evidence 4: In patients with Crohn’s disease and evidence of fistulizing disease, we recommend the use of anti-TNF therapy, to induce symptomatic response. GRADE: Strong recommendation, very low-quality evidence. 5: In patients with Crohn’s disease and evidence of fistulizing disease who have achieved symptomatic response on anti-TNF therapy, we suggest the use of continued therapy, to achieve and maintain complete remission. GRADE: Conditional recommendation, low-quality evidence. 6: In patients with Crohn’s disease and evidence of fistulizing disease, when starting anti-TNF therapy, we suggest it be combined with a thiopurine or methotrexate over monotherapy to optimize pharmacokinetic parameters. GRADE: Conditional recommendation, low-quality evidence for infliximab, very low-quality evidence for adalimumab” (p 6) Notes: Strength of recommendations: “…vote of ≥75% of participants needed to classify a statement as “strong” (recommended); if this threshold was not met, the statement defaulted to “conditional” (p 3) Quality of evidence: “Evidence of very-low quality: Any estimate of effect is very uncertain” (p 3) “Evidence of low-quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate” (p 3) | Not applicable |
About the Series
CADTH Rapid Response Report: Summary with Critical Appraisal
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
Sequencing of pharmacological management of Crohn’s disease and Ulcerative Colitis: a review of guidelines. Ottawa: CADTH; 2019 Jan. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governmentsor any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.