Except where otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-sa/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Patient population. Adults with, or at risk for Type 2 Diabetes
Objectives. To reduce morbidity and mortality by improving adherence to important recommendations for preventing, detecting, and managing diabetic complications.
Key points
Prevention. Type 2 diabetes may be delayed or prevented through diet, exercise, and pharmacologic interventions. [IA]
Screening. Consider screening every 3 years, beginning at age 45, or annually at any age if BMI ≥25 kg/m2 [IID], history of hypertension [IIB], gestational diabetes [IC], or other risk factors.
Diagnosis. Diagnosis is made by (1) an A1c ≥6.5%, (2) a fasting glucose ≥126 mg/dL, (3) a 2h post 75 gm glucose load glucose of ≥200 mg/dL, or (4) a random glucose ≥200 mg/dL with symptoms, confirmed by a repeat or second test. Diagnostic criteria are shown in Table 1. An abbreviated differential diagnosis of diabetes is shown in Table 2. It is important to recognize diabetes types due to insulin deficiency as the pathophysiology directs treatment recommendations. An A1c of ≥6.5%, confirmed by second test, is diagnostic of diabetes. Alternatively, diabetes is diagnosed by two separate fasting glucose tests ≥126 mg/dL; with symptoms, a glucose ≥200 mg/dL confirmed on a separate day by a fasting glucose ≥126 mg/dL; or 2-hour postload glucose ≥200 mg/dL during an oral glucose tolerance test (OGTT). [IIC].
Treatment. Essential components for diabetes treatment include: diabetes self-management education and support, lifestyle interventions, and goal setting (Table 3); glycemic management (Tables 4-7); and pharmacologic management of hypertension (Table 8) and hyperlipidemia.
Screening for comorbidities and complications. Routine screening and prompt treatment for cardiovascular risk factors (hypertension, hyperlipidemia, tobacco use) and for microvascular disease (retinopathy, nephropathy, neuropathy) are recommended in the time frames below.
Treatment of comorbidities and complications. Table 9 summarizes Management of risk factors and complications. Diet, exercise, and pharmacologic interventions should be initiated for: Hypertension [IA], Hyperlipidemia [IA], Cardiovascular risk reduction [IA], Diabetes complications as indicated.
| Each regular diabetes visit | Annually |
|---|---|
|
|
| Special considerations: Pregnancy. Preconception counseling and glycemic control targeting a normal A1c in women with diabetes mellitus is essential to reduce the risk of congenital malformations and results in optimal maternal and fetal outcomes. [IB] | |
Table 1
Diagnosis of Diabetes: Diagnostic Tests and Glucose Values.
Table 2
Abbreviated Differential Diagnosis of Diabetes.
Table 3
Self-Management Topics.
Table 4Targeting and Monitoring Glycemic Control in Patients with Diabetes Mellitus
| Target A1c: assess individual’s risks and benefits of treatment. | |||
| Factors heightening risk of tight control (hypoglycemia) | Factors limiting benefit of tight control | ||
| History of severe hypoglycemia (inability to treat without assistance). | Severe comorbidities (eg, end-stage cancer, severe heart failure). | ||
| Hypoglycemia unawareness. | |||
| Advanced cardiovascular, cerebrovascular and especially renal disease. | Limited life expectancy (<10 years) | ||
| Autonomic neuropathy (especially cardiac). | Adverse effects of treatment | ||
| Functional or cognitive limitations that cause inability to safely carry out treatment regimen. | |||
| If neither factors heightening risk nor limiting benefit of tight control: prevent long-term complications and early mortality. | |||
| <6.5% | Consider for:
| ||
| ≤7% | General target. | ||
| If factors heightening risk of tight control (hypoglycemia) | |||
| <7% | Consider if achievable with medications that do not incur risk of hypoglycemia (acarbose, metformin, TZDs, GLP-1s, or SGLT-2s). IA | ||
| <8% | General target if using medications increasing risk of hypoglycemia. IA | ||
| If factors limiting benefit of tight control: minimize symptoms of hyperglycemia and controlling glucose as well as possible without incurring side effects or excessive treatment burden. | |||
| <8% | General target. IA | ||
| <8.5% | Consider if multiple coexisting chronic illnesses, cognitive impairment, or functional dependence. IIC | ||
| <9% | Consider for very sick patients with limited life expectancy in order to avoid acute symptoms. IIE | ||
If A1c is above goal:
| |||
Reassess Glycemia:
| |||
Table 5Comparisons of Oral Agents for Glycemic Control in Patients with Type 2 Diabetes
| Generic | Brand Name | A1c Reduc-tionb | Δ Weight | Hypo-glycemia | Strength (mg) | Initial dose (mg) | Max Daily dose (mg) | Usual daily dose (mg) | Costd 30 days(range) Generic ($) Brand | Renal Dose Adjust | Other Side Effects/Precautions | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biguanide | ||||||||||||
| Metformin | Glucophage IR | ⇩⇩ | ⬄/⇩ | Nonea | 500, 850,1000 | 500 daily or 850 with meal | 2550 | 1500 – 2000 2x daily | $22-43 | $3674 | Contraindicated with eGFR <30 mL/min/1.73 m2. Starting metformin in patients with an eGFR between 30-45 mL/min/1.73 m2 is not recommended. | GI side effects - GERD, nausea, diarrhea. Annual eGFR recommended, more often if at risk of developing renal impairment or have existing DKD. B12 deficiency |
| Metformin extended release | Glucophage XR | 500, 750, 1000 | 500 to 1000 daily with evening meal | 2000 | 1500-2000 daily or divided | |||||||
| Oral incretin mimetic Glucagon-like Peptide 1 Receptor Agonist (GLP1 RA) h | ||||||||||||
| Semaglutide | Rybelsus | ⇩ | ⇩⇩ | Nonea | 3, 7, 14 | 3 daily | 14 | 3-14 daily | NA | $834 | None | Increased amylase and lipase, nausea, vomiting, diarrhea, abdominal pain, dyspepsia, flatulence, GERD, cholelithiasis Pancreatitis FDA black box warning: risk of thyroid C-cell tumor in rodents. |
| Dipeptidyl peptidase 4 (DPP4) Inhibitorg,h | ||||||||||||
| Sitagliptin | Januvia | ⇩ | ⬄ | Nonea | 25, 50, 100 | 5-100 daily | 100 | 100 | N/A | $412 | Adjust for eGFR <45 mL/min/1.73 m2 | Class-wide: pancreatitis (rare), joint pain (rare) angioedema (rare) |
| Saxagliptini | Onglyza | 2.5-5 | 2.5-5 daily | 5 | 2.5-5 daily | N/A | $416 | Adjust for eGFR <45 mL/min/1.73 m2 | Saxagliptin: increased risk of heart failure | |||
| Linagliptin | Tradjenta | 5 | 5 daily | 5 | 5 DAILY | N/A | $412 | None | ||||
| Alogliptin | Nesina | 6.25, 12.5, 25 | 25 daily | 25 | 25 QD | NA | $405 | Adjust for CrCl <60 ml/min | ||||
| Sodium-glucose cotransporter 2 (SGLT2 Inhibitor) j | ||||||||||||
| Canagliflozin | Invokana | 100, 300 | 100 daily | 300 | 100 daily before first meal | NA | $476 | Adjust for eGFR <60 mL/min/1.73 m2 | Canaglflozin: lower limb amputations, bone fractures, hyperkalemia | |||
| Dapagliflozin | Farxiga | 5, 10 | 5 daily | 10 | 5 in AM | NA | $465 | Dapagliflozin, Empagliflozin, Ertugliflozin: Adjust for eGFR <45 mL/min/1.73 m2 Not recommended for eGFR <30 mL/min/1.73 m2 | Class-wide: hypotension, risk of volume depletion, diabetic ketoacidosis, urinary tract infection increases LDL, urosepsis, genital mycosis, polyuria, nausea, AKI, risk of Fournier’s gangrene | |||
| Empagliflozin | Jardiance | ⇩ | ⇩ | None a | 10, 25 | 10 daily | 25 | 10-25 daily | NA | $465 | To avoid any potential DKA, discontinue before scheduled surgery. | |
| Ertugliflozin | Steglatro | 5, 15 | 5 daily | 15 | 5-15 daily | NA | $319 | |||||
| Sulfonylureas (2nd Generation) e | ||||||||||||
| Glimepiride | Amaryl | ⇩⇩ | ⇧ | ⇧ | 1, 2, 4 | 1-2 daily | 8 | 4 daily | $17-25 | $172-526 | Glimepiride: Dose adjust for renal patients, consider alternative if eGFR <15mL/min | Glimepiride and glyburide: avoid in elderly patients due to risk of prolonged hypoglycemia. |
| Glipizide | Glucotrol | 5, 10 | 2.5, 5 daily | 40 | 10 - 20 divided (2x daily) | $8-13 | $167-330 | Glipizide and glipizide XL: preferred in class for renal patients given greater hepatic metabolism | ||||
| Glipizide XL | Glucotrol XL | 2.5, 5, 10 | 5 daily | 20 | 5 - 20 daily or divided (2x daily) | $15-50 | $89-330 | |||||
| Glyburide | Diabeta, Micronase | 1.25, 2.5, 5 | 2.5-5 daily | 20 | 5 - 20 daily or divided (2x daily) | $15-50 | N/A | Glyburide- not recommended in DKD | ||||
| Glyburide, micronized | Glynase | 1.5, 3, 4.5, 6 | 0.75-3 daily | 12 | 3 - 12 daily or divided (2x daily) | $11-45 | $150-500 | |||||
| Thiazolidinedione f | ||||||||||||
| Pioglitazone | Actos | ⇩⇩ | ⇧⇧ | Nonea | 15, 30, 45 | 15-30 daily | 45 | 15-45 daily | $14-16 | $420-640 | None | CHF, macular edema, LE edema, fractures, bladder cancer |
| Alpha-glucosidase inhibitor | ||||||||||||
| Acarbose | Precose | ⇩ | ⬄ | Nonea | 25, 50, 100 | 25 daily with meal | 300 | 50 - 100 TID before meals | $46-55 | $98-120 | Contraindicated for CrCl <25 ml/min or Scr ≥2 | GI side effects - flatulence, nausea, diarrhea, elevated LFTs |
| Non-sulfonylurea insulin secretagogues | ||||||||||||
| Repaglinide | Prandin | ⇩ | ⇧ | ⇧ | 0.5, 1.2 | 0.5 with meals | 16 | 0.5 - 4 AC daily to QID | $55 | $600 | Dose adjustment for CrCl <40 ml/min | Rare |
| Nateglinide | Starlix | 60, 120 | 60–120 with meal | 360 | 60 - 120 AC daily to QID | $81-87 | $321-334 | None | ||||
- *
Combination oral products are available.
- a
When used as monotherapy
- b
A1c reduction is dose dependent
- c
In animal models
- d
Cost = Average Wholesale Price minus 10%. AWP from Red Book Online 5/17. For generic drugs, Maximum Allowable Cost plus $3 from BCBS of Michigan MAC List, 5/17.
- e
Second generation sulfonylureas have a better safety profile compared to first generation sulfonylureas.
- f
Pioglitazone is preferred over rosiglitazone because of its cardiovascular risks. However, the FDA recently cautioned that pioglitazone has been associated with increased risk of bladder cancer after 12 months of use. Physicians should avoid pioglitazone in patients with active bladder cancer and with caution in patients with a prior history of bladder cancer.
- g
When administered with a sulfonylurea, a lower dose of the sulfonylurea may be required.
- h
DPP-4 and GLP-1 RA are considered therapeutic duplicates. Do not use DPP4 and GLP-1 RA together.
- i
Consider discontinuing saxagliptin in patients who develop heart failure.
- j
Assess volume status and renal function before initiation. Correct volume depletion before initiation.
Table 6Comparisons of Injectable Agents for Glycemic Control in Patients with Type 2 Diabetes
| Generic | Brand Name | A1c Reductionb | Δ Weigh | Hypoglycemia | Strength | Initial dose | Max dose | Costd 30 days ($) | Renal Dose Adjust | Other Side Effects/Precautions |
|---|---|---|---|---|---|---|---|---|---|---|
| Glucagon-like Peptide 1 Receptor Agonist (GLP1 RA): incretin mimetics h | ||||||||||
| Exenatide | Byetta | ⇩⇩ | ⇩⇩ | Nonea | 5, 10 mcg | 5 mcg BID | 10 mcg BID | $721 | Exenatide: contraindicated for CrCl <30mL/min | Class-wide: nausea, vomiting, diarrhea, constipation, abdominal pain, pancreatitis, |
| Exenatide extended-release | Bydureon, Bydureon BCise | 2 mg | 2mg once weekly | 2mg once daily | $675 | Exenatide XR: contraindicated for CrCl <45mL/min | Exenatide, exenatide extended release: injection site reaction/nodule | |||
| Liraglutide | Victoza | 0.6,1.2, 1.8 mg | 0.6 mg daily | 1.8 mg daily | $538 | Liraglutide, dulaglutide, semaglutide: No specific guideline. Use caution when initiating or escalating doses | Exenatide, exenatide extended release, liraglutide: headache | |||
| Dulaglutide | Trulicity | 0.75, 1.5, 3, 4.5 mg | 0.75 mg once weekly | 4.5 mg once weekly | $730 | Lixisenatide: contraindicated for CrCl <15mL/min | Liraglutide, dulaglutide, exenatide extended release: risk of thyroid C-cell tumorsc | |||
| Lixisenatide | Adlyxin | 10, 20 mcg | 10 mcg daily | 20 mcg daily | $334 | |||||
| Semaglutide | Ozempic | 0.25, 0.5, 1 mg | 0.25 mg weekly | 1 mg daily | $943 | |||||
| Generic | Brand Name | A1c Redu-ctionb | Δ Weight | Hypo-glycemia | Onset of action | Peak of Action | Duration of Action | Costd 30 days ($) | Renal Dose Adjust | Other Side Effects/Precautions |
| Ultra Rapid-acting insulin | ||||||||||
| Aspart Lispro-aabc | Fiasp Lyumjev | ⇩⇩⇩ | ⇧ | ⇧⇧ | 5-15 min | 1.5-2hours | 5-7 hours | None | Rare | |
| Rapid-acting insulin | ||||||||||
| Lispro | Humalog, Admelog | ⇩⇩⇩ | ⇧ | ⇧⇧ | 15 min | 0.5-2.5 hours | 3-5 hours | $574 | ||
| Aspart | NovoLog | 15 min | 1-3 hours | 3-5 hours | $575 | None | Rare | |||
| Glulisine Insulin human | Apidra | 20 min | 1-2 hours | 5-6 hours | $532 | Insulin human: pulmonary toxicity; requires normal PFTs prior to prescription. | ||||
| Short-acting insulin | ||||||||||
| Regular | Humulin R, Novolin R | ⇩⇩⇩ | ⇧ | ⇧⇧ | 30-60 min | 2-3 hours | 3-6 hours | $150 | None | Rare |
| Intermediate insulin | ||||||||||
| NPH | Humulin N, Novolin N | ⇩⇩⇩ | ⇧ | ⇧⇧ | 2-4 hours | 4-10 hours | 10-16 hours | $150 | ||
| Detemir | Levemir | 3-4 hours | 6-8 hours | 6-23 hours | $435 | None | Rare | |||
| Long acting insulin | ||||||||||
| Glargine | Lantus, Basaglar Semglee | ⇩⇩⇩ | ⇧ | ⇧⇧ | 2-4 hours | None | 20-24 hours | $405 | None | Rare |
| Glargine U300 | Toujeo Toujeo Max | 6 hours | None | Up to 24 hrs | ||||||
| Degludec | Tresiba | 1 hour | 9 hours | Up to 42 hours | $480 | |||||
| Combination Insulin | ||||||||||
| Intermediate- and short/rapid-acting mixtures | 75/25 Insulin lispro protamine/insulin lispro (Humalog Mix 75/25) | Varies according to types and percentages of insulin | $570 | None | Rare | |||||
| 50/50 Insulin lispro protamine/insulin lispro (Humalog Mix 50/50) | $310 | |||||||||
| 70/30 Insulin aspart protamine/insulin aspart (Novolog Mix 70/30) | $310 | |||||||||
| Long-acting and rapid-acting mixture | 70/30 NPH/regular (Humulin 70/30, Novolin 70/30) | See individual agent profiles | ||||||||
| 70/30 Degludec/aspart (Ryzodeg) | ||||||||||
| Concentrated, intermediate acting | U500 regular (Humulin R) | 30 minutes | 1.5-3.5 hours | Up to 24 hours | $290 | |||||
- *
Combination injectable products are available.
- a
When used as monotherapy
- b
A1c reduction is dose dependent
- c
In animal models
- d
Cost = Average Wholesale Price minus 10%. AWP from Red Book Online 5/17. For generic drugs, Maximum Allowable Cost plus $3 from BCBS of Michigan MAC List, 5/17.
- e
Second generation sulfonylureas have a better safety profile compared to first generation sulfonylureas.
- f
Pioglitazone is preferred over rosiglitazone because of its cardiovascular risks. However, the FDA recently cautioned that pioglitazone has been associated with increased risk of bladder cancer after 12 months of use. Physicians should avoid pioglitazone in patients with active bladder cancer and with caution in patients with a prior history of bladder cancer.
- g
When administered with a sulfonylurea, a lower dose of the sulfonylurea may be required.
- h
Assess volume status and renal function before initiation. Correct volume depletion before initiation.
- i
Consider discontinuing saxagliptin in patients who develop heart failure
Table 7Comparisons of Agents for Glycemic Control on ASCVD, CHF and DKD in Patients with Type 2 Diabetes
| Cardiovascular and Renal Benefits | ||||
|---|---|---|---|---|
| Drug Class | Medication | ASCVD | CHF | DKD progression |
| Biguanides | Metformin | Potential Benefit | Neutral | Neutral |
| SGLT-2 inhibitors | Canaglifozin, Dapagliflozin, Empagliflozin Ertugliflozin | Benefit (Empagliflozina, Canagliflozin) | Benefit (Canaglifozin, Dapagliflozinb, Empagliflozina) | Benefit (Canaglifozinc, Dapagliflozin, Empagliflozin) |
| GLP-1 agonists | Exenatide, Liraglutide, Exenatide extended release, Dulaglutide, Lixisenatide, Semaglutide | Benefit: Liraglutidea, Dulaglutidea, Semaglutidea Neutral: Lixisenatide, Exenatide extended release | Neutral | Benefit on renal end points in CVOTs, driven by albuminuria outcomes: Liraglutide, Semaglutide, Dulaglutide |
| DPP-4 inhibitors | Sitagliptin, Saxagliptin, Linagliptin, Alogliptin | Neutral | Potential risk: saxagliptin | Neutral |
| Thiazolidinediones | Pioglitazone | Potential benefit | Increased risk | Neutral |
| Sulfonylureas | Glimepiride, Glipizide, Glyburide | Neutral | Neutral | Neutral |
| Insulin | All | Neutral | Neutral | Neutral |
- a
FDA approved for CVD benefit
- b
FDA approved for heart failure indication
- c
FDA approved for DKD indication
Table 8
Steps in Pharmacologic Treatment of Hypertension in Patients with Diabetes Mellitus.
Table 9
Prevention, Screening, and Treatment of Complications in Patients with Diabetes Mellitus.
Footnotes
- *
Strength of recommendation:
I = generally should be performed; II = may be reasonable to perform; III = generally should not be performed.
Level of evidence supporting a diagnostic method or an intervention: A=Systematic review of randomized controlled trials; B=Randomized controlled trials; C=Systematic review of non-randomized controlled trials; group observational studies; D=Individual observation descriptive studies; E=Expert opinion
Clinical Problem: Prevalence and Outcomes
Definitions. Type 2 Diabetes is defined as chronic hyperglycemia resulting from either decreased insulin secretion, impaired insulin action, or both in the absence of Type 1 diabetes (autoimmune destruction of the pancreatic beta cell), Type 3c diabetes (pancreaticogenic diabetes) or other specific type (Table 2). Classically, type 2 diabetes occurs in the older, obese patients in the setting of strong family histories of diabetes and in association with other components of the metabolic syndrome.
Prevalence. About 10.5% of the U.S population has diabetes, with 85% of these people having type 2 diabetes. In addition, 34.5% of the adult US population has prediabetes. The prevalence of diabetes increases with age, with over 26.8% of those ≥65 years old having type 2 diabetes. Non-Caucasians have a prevalence of type 2 diabetes mellitus that is 2 to 6 times greater than that of Caucasians.
Increasing obesity in the general population is driving a world-wide epidemic of type 2 diabetes. Obesity is also increasing the prevalence of type 2 diabetes at younger ages. Type 2 diabetes is now present in 4.2% of those aged 20 to 39 years.15
Obesity is also affecting characteristics that previously distinguished populations likely to have type 2 or type 1 diabetes. Type 2 diabetes typically occurred in patients over 30 years old and weighing ≥120% of ideal body weight, while type 1 diabetes occurred in patients under age 30 and weighing <120% of ideal body weight. In addition to obesity lowering the age at which type 2 diabetes is commonly seen, population weight increases are resulting in a greater proportion of patients with type 1 diabetes being overweight.
Inadequate screening and treatment. Type 2 diabetes often has a long (up to 10 years) pre-symptomatic phase, and national studies suggest that approximately a third of subjects with type 2 diabetes are unaware that they have the disease.16–19 Studies suggest that early treatment can reduce long term complications. Furthermore, screening for and treatment of co-morbidities and early diabetic complications is effective in reducing the incidence of end-stage complications. However, implementation rates of recommended screening procedures are low, leading to ineffective and/or delayed treatment of diabetes, and its comorbidities and complications. This, in turn, increases the costs of medical care and adversely affects quality of life.
Outcomes. Despite significant improvements in care, diabetes continues to have high morbidity and mortality. Based on data from 2016 reported by the CDC, diabetes remained the leading cause of blindness in working age adults, with 11.7% of patients with diabetes affected by visual impairment.15 Diabetic kidney disease (DKD) occurs in 37% of diabetic patients. In the United States, 38.6% of ESRD cases were attributed to diabetes,15 making diabetes the leading cause of ESRD in the US. In 2016 alone, 5.6 of every 1000 patients with diabetes were admitted for a lower extremity amputation.15 This culminates in diabetes being the 7th most common cause of death in the US.
Rationale for Recommendations
Diabetes Prevention
Recommendations
Refer patient to a Diabetes Prevention program if screening test results are in the pre-diabetes range.
Provide resources for weight loss (goal of 5-7% reduction in baseline weight) if the patient is not able to, or is unwilling to participate in a Diabetes Prevention Program.
Prescribe Metformin 850 mg a day and increase to BID (as tolerated) if unable to lose weight.
Several large randomized controlled trials have demonstrated that lifestyle modification programs delay or prevent type 2 diabetes in patients who have impaired glucose tolerance.20 One possible additional benefit of screening for diabetes is the identification of people with impaired glucose tolerance. Those with a fasting glucose of 100-125 mg/dL, A1c 5.7-6.4, or a 2-hour OGTT of 140-199 mg/dL are considered at risk for diabetes.21 (A random glucose of 130-199 mg/dL is abnormal and further testing is indicated, eg, fasting glucose, OGTT, or hemoglobin A1c). Large randomized controlled trials from China, Finland, India, and the United States have shown that programs targeting modest improvements in diet and physical activity (7% reduction in body weight and 150 minutes of brisk walking per week) can reduce the risk of progression from impaired glucose tolerance (IGT) to diabetes by 42-58%.19 The intensive lifestyle intervention tested in the Diabetes Prevention Program (DPP) was expensive, but cost-effective. A large number of translational studies have shown that both group-based DPP22 and online DPP23 are effective and less expensive.
A number of medications have also been shown to decrease progression to diabetes in pre-diabetic patients. In the Diabetes Prevention Program, metformin 850 mg twice daily demonstrated a 31% risk reduction in progression from IGT to diabetes, about half as effective as lifestyle. Other medications that have shown efficacy in diabetes prevention include pioglitazone, acarbose, liraglutide, and orlistat, either alone or in combination with lifestyle interventions.24 These studies suggest that a pharmacologic approach to diabetes prevention may also be feasible, but lifestyle interventions remain the standard of care. For those who cannot or will not engage in a lifestyle change program, metformin is a reasonable alternative to prevent diabetes.
Screening for diabetes
Recommendations
Consider screening for diabetes every 3 years beginning at age 45, or annually at any age if BMI ≥25 kg/m2, history of hypertension, gestational diabetes or other risk factors.
Studies of screening for diabetes do not clearly suggest that screening will lead to significant improvements in diabetes outcomes; therefore, the effectiveness (or cost–effectiveness) of screening on a population-wide basis is not clear. The value of screening for diabetes increases when adding in the benefits of detecting prediabetes and intervening with effective diabetes prevention strategies. The American Diabetes Association (ADA) recommends screening be considered at least at 3-year intervals, beginning at age 45 in people with a BMI >25 (Asians >23) and with one other risk factor for diabetes. Individuals with multiple risk factors for diabetes may be considered for screening at earlier ages. Individuals with hypertension (>135/80) should be screened for diabetes (USPSTF level B recommendation). In adults who have hypertension and diabetes who are at higher risk for cardiovascular disease, achieving lower blood pressure targets reduces the incidence of cardiovascular events and cardiovascular mortality and justifies screening. Individuals who have been diagnosed with pre-diabetes benefit from diabetes prevention interventions and should be tested annual for progression to diabetes.
Screening every 3 years may be reasonable for other at-risk people, including those with obesity, a history of gestational diabetes mellitus, polycystic ovarian disease, or a strong family history of diabetes. Women planning pregnancy may also benefit from screening as Type 2 diabetes is increasingly common in this population and identifying and treating undiagnosed diabetes preconception can prevent congenital malformations. If a provider elects to screen for diabetes, the tests outlined in the “diagnosis” section should be used (Table 1).
Depression
Elevated depressive symptoms and depressive disorders affect 25% of patients with type 2 diabetes,25 and increase the risks of hyperglycemia, insulin resistance, and micro- and macrovascular complications.26
Providers should consider screening diabetes patients for depressive symptoms annually, and whenever there are major changes in medical status (eg, new complications, treatment intensification, etc).27
Depression screening can be done with the PHQ-2. For example: “Over the last 2 weeks, how often have you been bothered by any of the following problems?”.
- “Little interest or pleasure in doing usual things?”
- “Feeling down, depressed or hopeless?”
Patients who respond “yes” to either question should be further evaluated using the full PHQ-9 to determine whether they meet criteria for a depressive disorder. See UMHS clinical guideline on depression for more information on the PHQ-9.
Both psychotherapy and antidepressant medication are moderately effective for depression, and cognitive behavior therapy (CBT) has additional benefits for glycemic control.28
If PHQ-9 total ≥10: Patients should be treated for depression with pharmacotherapy and/or referral to a behavioral health specialist for cognitive behavioral therapy (CBT), or antidepressant management. See UMHS clinical guideline on depression for full details on depression treatment.
PHQ-9 totals of 5-9: Consider depression treatment, counseling/psychotherapy, or watchful waiting, and repeat PHQ-9 at follow-up.
Disordered eating behaviors
Consider screening for disordered/disrupted eating or omission of insulin doses when hyperglycemia is unexplained. Patients with diagnosable eating disorder can be referred to a specialty behavioral health provider.
Diabetes-related distress
Diabetes distress refers to significant negative psychological reactions to having diabetes. This is distinct from depressive and anxiety disorders, affects up to 45% of patients, and is linked with poor glycemic control, medication non-adherence, and poor dietary and exercise behaviors.
Routinely monitor for diabetes distress, especially when treatment targets are not met and/or at the onset of diabetes complications.27
Screening can be accomplished with the Diabetes Distress Scale – 2 (DDS-2).29 Patients rate how much each problem has distressed or bothered them over the past month using the following 6-point scale: 1-Not a problem, 2-Slight problem, 3-Moderate problem, 4-Somewhat serious problem, 5-Serious problem, 6-Very serious problem.
- Feeling overwhelmed by the demands of living with diabetes.
- Feeling that I am often failing with the prescribed diabetes regimen.
A total score of 6 or more indicates significant diabetes distress and is an indication for intervention.
Patients who screen positive for diabetes distress should first be offered diabetes education to address the areas of self-management that are most relevant to their personal concerns and health outcomes. Those who do not respond to education should be referred to a specialty behavioral health provider familiar with diabetes self-management.
Diagnosis
Recommendations
An A1c of 6.5% or greater confirmed by another test on a second day is diagnostic of diabetes.
Various methods can be used to diagnose diabetes according to the American Diabetes Association (ADA). One common test for diagnosis is the A1c. Diabetes is diagnosed if A1c is 6.5% or higher. This cut point is specific, but not sensitive, and thus individuals with A1c 6.0% - 6.4% may meet criteria for diabetes using fasting glucose or OGTT tests. Advantages of the A1c as a diagnostic test include convenience (as it does not require fasting) and a highly standardized assay. A1c may not be accurate for patients with hemoglobinopathies, thalassemia, hemolysis, blood loss, or iron deficiency.
Alternatively, a FPG or OGTT may also be used to diagnose diabetes. The diagnosis can be made if a FPG level is ≥126 mg/dL (7.0 mmol/L), a repeat verification is necessary. Diabetes may also be diagnosed on the basis of symptoms (polydipsia, polyuria, unintentional weight loss) and elevated glucose level (≥ 200 mg/dL). The OGTT is a reasonable diagnostic alternative, and in the view of many experts remains the diagnostic test of choice, however, may be inconvenient for patients. A 2-hour glucose level of ≥ 200 mg/dL is diagnostic for diabetes. All tests (except for the OGTT) should be repeated or confirmed with alternative tests on a separate day.
While most patients diagnosed with diabetes are classified as having Type 2 diabetes, it is important to consider other forms of diabetes, as this can affect prognosis and treatment. Type 1 diabetes occurs at relatively similar rates across the lifespan, and ultimately accounts for between 5-10% of diabetes cases. Patients aged 30 to 60 years account for 4% to 14% of newly diagnosed Type 1 diabetes.30,31
The dramatically increasing incidence of Type 2 diabetes in the 30 to 60 year age group confounds the diagnosis and contributes to the delay of insulin initiation in 38% of those with Type 1. Furthermore, nearly half of those with Type 1 in this age group self-report that they have Type 2 diabetes.32
Consider Type 1 diabetes in patients with personal/family history of autoimmune disease including thyroid, celiac, and B12 deficiency, normal or low BMI, weight loss preceding diagnosis, exposure to check point inhibitor medications (ipilimumab, nivolumab, etc.), or severe hyperglycemia requiring early initiation of insulin.
Pancreaticogenic diabetes (Type 3c) accounts for roughly 8% of diabetes cases.33 Consider pancreaticogenic diabetes in patients with personal/family history of chronic pancreatitis, pancreatic cancer, cystic fibrosis or hemochromatosis, personal history of alcoholism, chronic GI complaints, elevated AST/ALT, normal or low BMI, weight loss preceding diagnosis, or severe hyperglycemia requiring early initiation of insulin.
There are many rare forms of diabetes, which combined, account for up to 3% of diabetes cases. These include MODY 1-11, PNDM, Wolfram’s syndrome, lipodystrophic diabetes, mitochondrial forms of diabetes, myotonic dystrophy, etc. Consider unusual forms of diabetes in patients with evidence of congenital syndromes, early hearing or visual loss, normal or low BMI, unusual body fat distribution, severe obesity, severe hypertriglyceridemia, and early age of diagnosis.
Recognizing Type 1 diabetes, pancreaticogenic diabetes and other less common forms at the time of diagnosis will ensure that patients who require insulin receive it promptly, avoid admissions for diabetic ketoacidosis (DKA), and reduce morbidity.
In patients with Type 2 diabetes consider contributing factors, such as chronic liver disease, medications, endocrinopathies and monoclonal gammopathy, which may be driving insulin resistance. Hepatitis C is present in over 5% of patients with Type 2 Diabetes. Treatment for Hepatitis C has been shown to improve glycemic status.34 Numerous medications have been shown to increase insulin resistance and contribute to Type 2 diabetes. Careful review of the medication list is essential. Endocrinopathies, most commonly Cushing’s syndrome and Hyperaldosteronism, contributes to over 2% of cases of Type 2 diabetes.35 Monoclonal gammopathies are present in roughly 20% of patients with Type 2 diabetes and neuropathy.36
Treatment
Recommendations
Treatment goals include reducing glycemia, controlling blood pressure, reducing cardiovascular disease risk, and preventing complications.
Glycemia targets should be individualized. Weight loss, exercise and diet changes can dramatically improve glycemia.
Medications that improve long-term outcomes (metformin, SGLT2i, and GLP1RA) are preferred.
Cardiovascular disease prevention is critical including adequate blood pressure control, smoking cessation, and statins.
Screening, self-management and treatment for diabetic complications including: retinopathy, lower extremity ulcers, and diabetic kidney disease also improves long term outcomes.
Diabetes Self-Management
Most of the diabetes management takes place outside of the doctor’s office. Diabetic patients require daily self-management. Individuals may be instructed to dramatically change their diet, engage in a regular exercise program, take multiple medications, monitor their blood glucose and blood pressure, and check their feet daily. The burden of self-managing diabetes can be overwhelming, particularly for those with limited psychosocial supports or pre-existing mental health problems. Consider a referral to an intensive diabetes self-management education class (DSME) for those who may be struggling with self-management (newly diagnosed or persistent poor control). In addition to DSME, patients also need on-going self-management support in order to sustain improvements gained during DSME. Table 3 summarizes self-management topics that clinicians should address at each visit and annually.
- Diabetes self-management education is effective for improving psychosocial and health outcomes (including A1c) and for reducing costs.
- Traditional knowledge based DSME is essential but not sufficient for sustained behavior change. People with diabetes need on-going clinical, psychosocial and behavioral diabetes self-management support (DSMS).
- No single strategy or programmatic focus shows any clear advantage, but interventions that incorporate behavioral and affective components are more effective.
- DSME is more effective when tailored to the patient’s preferences, social and cultural situation.
- DSME is most effective when coupled with appropriate care and reinforcement by all health care professionals and on-going DSMS.
- Organizational interventions that improve diabetes self-management include computerized tracking systems, regular recall and review of patients, the addition of patient-centered educational and counseling approaches, and behavioral goal-setting.
- On-going self-management support can be effectively delivered by appropriately trained panel managers, or care managers, dieticians, nurses, Pharm. Ds, remotely delivered eHealth programs, peer support, and group or cluster visits.
Meal planning. Meal planning is recommended for all stages of diabetes.
Medication adherence. Medication adherence is associated with better glycemic control, lower rates of hospitalization and mortality, and lower healthcare costs.37 However, only about 68% and 59% of patients adhere adequately to oral agents and insulin, respectively. Non-adherence is more likely among patients who are younger, female,38 socioeconomically disadvantaged,39,40 of low health literacy, and depressed.41
Providers should screen patients who have persistently poor glycemic control, recent regimen intensification, or other risk factors. Note that patients seldom report non-adherence spontaneously. Clinicians should inquire non-judgmentally by acknowledging the widespread difficulty of taking medication as prescribed,42 eg, “Most people have trouble taking medication exactly as prescribed, every dose and every day. Do you ever have any difficulty taking your [diabetes medication] exactly as prescribed?”
Because such direct queries are more specific than sensitive, clinicians should maintain a low threshold for suspecting non-adherence, and further inquire about reasons for noanadherence.42 Eg,
- “Do you ever forget to take your [diabetes medication]?”
- “Do you believe that your [diabetes medication] is effective enough?”
- “Do you ever worry that your [diabetes medication] may be harmful?”
- “Are you burdened by your medication costs?”43
Educational interventions with behavioral support can be effective in addressing non-adherence, although they often need to be extended for months. For persistent non-adherence, referral to a diabetes educator or behavioral health clinician is usually indicated.44
Practical/logistical issues
One of the most common barriers to adherence is the ability to obtain testing supplies and medications by both patients and physicians. There are several practical considerations.
Diabetes medications are frequently the target of the insurance companies’ ever-changing formularies, resulting in unexpected changes to patients’ out-of-pocket expenses. Physicians often experience requests for a medication change (within and outside of drug class) and prior authorizations requests. Increased medication expenses often lead to patient non-adherence and/or financial hardship. Delays may occur in obtaining crucial medications while prescriptions are changed and/or prior authorizations are acquired. In order to minimize disruptions, ACUs should have processes and procedures in place to facilitate physicians, nurses, pharmacists, and other team members to identify financial barriers to medication adherence, update nursing protocols regularly regarding pre-approved medication substitutions, and employ an efficient and effective medication prior authorization procedure. (Nursing protocols can be found at http://www.med.umich.edu/i/acs/nursing/standingorders/standingorders_protocols.html)
Ordering diabetes testing supplies often represents an even greater challenge. Physicians are often not familiar with the pros and cons of various devices, and insurers often limit the available options, making writing specific prescriptions challenging. If a specific meter known for its accuracy is prescribed it may not be covered by insurance, unintentionally delaying care. If the meter choice is made by the DME, or pharmacy, the result may be a meter that is only within an acceptable margin of error 75% of the time. For a recent study of glucometers, please see: https://www.diabetestechnology.org/surveillance.shtml.
Most medications are covered by a patient’s pharmacy benefit, however 80% of insurance plans categorize diabetes testing supplies, including continuous glucose meters, and some insulin delivery devices (insulin pumps) as Durable Medical Equipment (DME). Like medications, the brand and type of diabetes testing supplies available under a plan changes from year-to-year, but there is the additional provision of where to obtain them (specific DMEs or Pharmacies). This frequently leads to patients presenting at a pharmacy with a prescription for test strips, only to be told that test strips are not covered under their pharmacy benefit plan; and failing to be told that they are covered under their DME coverage. It is a Michigan law that insurers cover testing supplies. (https://www.michigan.gov/difs/0,5269,7-303-12902_35510_92612_92613_92614_92867-497018--,00.html)
Most insurers, including Medicare, require more than a testing supply prescription for reimbursement. Most DME companies and some pharmacies fax or email staff questionnaires, or Certificates of Medical Necessity (CMNs) for completion. In an effort to eliminate unnecessary, duplicative paper work, Michigan Medicine’s Adult Diabetes Education program designed order sets in the Meds and Orders tab in MiChart. When signed, these are automatically sent to the selected DME company. Pharmacies require separate prescriptions for each item and a follow up fax for CMNs. The Adult Diabetes Education program continues to work with the remaining local and national DMEs and local pharmacies, in conjunction with the Endocrine Society and other academic institutions, to achieve greater acceptance of our electronic ordering of diabetes supplies.
There are two order sets in Michart. Diabetes DME- Testing supplies, (available for any provider to use to order glucometers, test strips, and lancets from a DME) and Diabetes DME – insulin pump and CGM supplies, (which is restricted to adult and pediatric diabetes education.) This restriction is due to the need to understand the various insurers requirements to obtain these devices and supplies. Please place a referral to diabetes education if you are wish to order an insulin pump or CGM for your patient.
Smoking cessation: Tobacco and E-Cigarettes
Recommendations
Assess routinely and advise to not use tobacco products, cigarettes or e-cigarettes.
Provide tobacco cessation education/counseling along with pharmacological treatment.
Discourage use of e-cigarettes as a way to stop smoking tobacco products.
Smoking and diabetes are synergistic risk factors for the development of atherosclerotic cardiovascular disease, microvascular disease, premature death and worsen glycemic control. Smoking is a risk factor for type 2 diabetes. Centers for Disease Control and Prevention recommends against the use of e-cigarettes either as a tobacco cessation product or as a recreational drug in light of deaths related to e-cigarettes. People with diabetes should be counseled regarding these risks, and all possible measures should be used to encourage patients to stop smoking. This includes enrollment in formal tobacco cessation programs and use of alternative nicotine delivery systems or pharmacologic therapies. Pharmacological therapy in addition to counseling is more effective than either treatment alone.
Glycemic Control
Hemoglobin A1c is the most commonly accepted measurement of long-term glycemic control, although factors such as hemolytic anemia and hemoglobinopathies can cause A1c measurement to be inaccurate.
Targets for glycemic control
Recommendations
A1c goals should be individualized.
Selection of an A1c goal should be based on:
- Factors that increase the risks associated with tight control; and
- Factors that limit the benefit of tight control. See Table 4 for more specific recommendations.
For CGM users, a reasonable goal is more than 70% time in range, with no more than 4% time below range.
- Both the range and the goal for TIR should be individualized, similarly to the A1c.
Targets for therapy of Type 2 diabetes have been evaluated in four large clinical trials: UK Prospective Diabetes Study (UKPDS), Action to Control Cardiovascular Risk in Diabetes study (ACCORD), Action in Diabetes and Vascular Disease Controlled Evaluation (ADVANCE) and The VA Diabetes Trial (VADT). An overview of each of these four trials is included in Appendix A. The intensive control group in each of the four trials achieved A1c levels ≤7.0. All four trials demonstrated reduction in microvascular outcomes with intensive glycemic control. All except ACCORD demonstrated reduction in macrovascular outcomes with intensive glycemic control. Therefore, many patients will benefit from a hemoglobin A1c goal less than 7%.
Conversely, a strict A1c goal of <7% may not be appropriate for many patients. ACCORD demonstrated an increase in macrovascular outcomes with intensive glycemic control. A 2019 metanalysis demonstrated that patients with pre-existing cardiovascular disease do not derive macrovascular benefit from strict control.45 Additionally, much of the microvascular benefit demonstrated by the four key trials derives from reduction in surrogate endpoints that may not be clinically important to patients such as microalbuminuria and fundoscopic appearance of early asymptomatic retinopathy.
Therefore, A1c targets should be individualized based on patient-specific factors. A1c targets should be discussed with patients. Providers should weigh patient-specific factors when considering glycemic goals (Table 4). Two important concepts that need consideration when selecting an A1c goal are 1. it takes years for symptomatic benefits to become apparent and 2. hypoglycemia is a potent predictor of mortality. Therefore, a number of factors may modify target levels. Factors that increase risk of hypoglycemia and its consequences include history of cardiovascular disease, previous history of hypoglycemia, hypoglycemia unawareness, and functional and/or cognitive limitations. Factors that limit life expectancy such as severe advanced comorbidities (advanced cancers, advanced heart failure) will limit the benefit of strict control. Furthermore, the burden, cost, and risk of the regimen needed to achieve a goal should also be considered.
Home Glucose Monitoring
Historically, evidence to support home blood glucose monitoring using finger stick glucose testing in type 2 diabetes treated with oral agents has been unconvincing. In addition to the fact that testing alone does not change glycemia, many patients find that finger sticks are painful, and thus is a common barrier to glucose self-monitoring.
More recently trials of continuous glucose monitoring (CGM), paired with behavioral coaching to help patients identify effective strategies for improving glycemia, have shown positive results.46 CGM provides patients with real time feedback about the carbohydrate load in their diet and about episodes of hypoglycemia. Newer technologies including low cost and user friendly CGM such as the Abbott Freestyle Libre® and the Dexcom G6® do not require finger sticks and provide more detailed glucose feedback, including glucose levels during sleep. CGM is increasingly being used in patients struggling to control their type 2 diabetes. Medicare insures CGM for people with Type 2 Diabetes who test 4 times a day. Monitoring with these devices is less expensive than covering lancets and test strips. As the cost of CGM continues to decrease, finger stick glucose testing will be replaced by CGM for most patients with type 2 diabetes.
CGM also provides summary measures including percent time in range, percent time above range and percent time below range. Typically, the recommended range is 70 to 180 mg/dL but this can be adjusted for patient specific factors in most CGM devices. A target of 70% time in range and no more than 4 % time below range, allowing some time for post-prandial glucose over 140 mg/dL has been recommended, but the evidence base for this recommendation is limited.
Glycemic management
Recommendations
Initiate Metformin, along with lifestyle modifications, as first line pharmacologic agent for type 2 diabetes.
- Continue metformin as long as it is tolerated and not contraindicated. Agents should be added to metformin.
Independent of A1c or metformin use:
- If patient is at high risk for ASCVD or has established ASCVD start a GLP1-RA
- If CKD or heart failure predominates, start a SGLT2i (or GLP-1RA if SGLT2i not available or safe)
Early combination therapy at treatment initiation can extend time to treatment failure in some patients.
- If there is evidence of catabolism (weight loss, hypertriglyceridemia, ketosis), symptoms of hyperglycemia, or A1c level is high (>10%) or blood glucose levels are high (≥ 300 mg/dL), initiate insulin.
- GLP-1 RA is preferred over insulin (unless noted above) if injectables are needed.
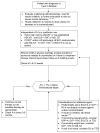
If additional pharmacological agents are needed, treat based on patient specific factors (Figure 1):
- To minimize hypoglycemia, consider SGLT2i, GLP-1 RA, DPP-4i, or TZD.
- If compelling need to minimize weight gain, consider GLP-1 RA or SGLT2i
- If cost is an issue, consider sulfonylurea or TZD
Evaluate for over-basalization with insulin therapy which includes basal dose more than ~0.5 IU/kg, high bedtime-morning or post-preprandial glucose differential, hypoglycemia, and high variability. Further individualize therapy and re-design regimen.
Do not delay intensification of treatment to meet target A1c goal.
Evaluate regimen every 6 weeks until target is achieved and then every 3 to 6 months thereafter.
In patients with type 2 diabetes, diet and physical activity are essential first line therapies, and many groups now recommend initiating metformin at diagnosis. A patient-centered approach should aid in the choice of subsequent agents (Figure 1). Patient factors to consider are co-morbidities (ASCVD and indictors of high ASCVD risk, DKD, and HF), risk of hypoglycemia, impact on weight, side effect profile, costs, and patient preference.
In general, if the patient has not achieved glycemic goal after 6 weeks of therapy at a maximal dose, the therapy should be considered inadequate. Numerous randomized controlled studies have shown SGLT2i and GLP-1 RA to have positive clinical outcomes on ASCVD, DKD and HF; therefore, SGLT-2i and GLP-1 RA should be considered if there is presence of ASCVD, DKD and/or HF. Choice of agents should have proven evidence for CVD, DKD, or HF benefits (Table 7).
For patients without established ASCVD, indicators of high ASCVD risk, HF, or DKD, there is little empiric evidence to guide next choice of agent. There are numerous trials comparing dual therapy with metformin alone but little comparative studies to support the use of one combination over the other. The GRADE study is the first large multicenter randomized control trial that compares the effects of sulfonylureas, DPP4 inhibitors, GLP-1 RA and insulin with metformin; however, until the trial is completed (estimated to be completed in 2022), the next agent should be chosen based on patient factors, cost, side effects, and patient preference.
Insulin therapy has the advantage of being effective and should be considered when hyperglycemia is severe or presence of catabolism. Once glucose toxicity has resolved, simplifying or changing therapy to other agents is possible. Otherwise, GLP-1 RA and SGLT2i’s are preferred over insulin when more glucose lowering is needed than can be achieved with metformin alone.
Figure 1 provides a stepwise summary of treatment recommendations. Table 5 summarizes cost, dosing and the medical advantages and disadvantages of the available oral agents to be considered for the management of type 2 diabetes. Table 6 summarizes the injectable agents to be considered for management of type 2 diabetes. Table 7 summarizes the impact pharmacological agents for type 2 diabetes has on ASCVD, DKD, and HF.
Metformin. The first recommended pharmacologic agent for type 2 diabetes is generally metformin. Metformin decreases hepatic glucose production, decreases intestinal absorption and increases peripheral glucose uptake and utilization by improving insulin sensitivity. It typically reduces A1c by 1-1.5%. Metformin has several characteristics that may provide secondary benefit:
- When used as a single agent, it rarely causes hypoglycemia and it does not cause weight gain.
- It appears to have favorable effects on lipid profiles and have cardiovascular protection. It has been associated with significant relative risk reduction in major adverse cardiovascular events (MACE) compared to sulfonylureas.
However, metformin has negative side effects and may not be tolerated by some patients.
- Nausea and diarrhea are the most common side effects; GI side effects are dose related. Metformin XR formulation may decrease diarrhea compared to the immediate release. The incidence of diarrhea for IR tablet is 12% to 53%; while ER tablet is 10%-17%.
- Metformin is associated with vitamin B12 deficiency which can lead to neuropathy. Recommend periodic testing for vitamin B12.
- Metformin is contraindicated in patients with eGFR <30 mL/min and should not be initiated in patients with eGFR between 30 and <45 mL/min. (ADA 2019) However, others recommend that in the absence of active kidney disease and/or conditions that predispose to hypoperfusion and hypoxemia (i.e. acute heart failure, dehydration), therapy may be initiated at half the usual initial dose with close monitoring.47 In patients taking metformin whose eGFR later falls below 45 mL/minute/1.73 m2, assess the benefits and risks of continuing treatment. Discontinue metformin if the patient’s eGFR later falls below 30 mL/minute/1.73 m2. Metformin is renally eliminated, so continuing metformin in acute renal failure has been associated with lactic acidosis, however, this complication is very rare.
Note that Metformin should discontinued at the time of, or before an iodinated contrast imaging in patients with an eGFR between 30 and 60 mL/minute/1.73 m2; in patients with a history of liver disease, alcoholism, or heart failure; or in patients who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure; restart metformin if renal function is stable.
When initiating metformin, start with 500 mg daily with food. Then increase the dose by 500 mg per week to 2000 mg per day as 2 or 3 divided doses as tolerated. Metformin therapy should be considered inadequate if the patient has not achieved his or her glycemic goal after four weeks of therapy at a maximum dose. Even after instituting pharmacologic therapy, careful attention should still be given to diet and physical activity.
In patients who are either not candidates for metformin therapy or have failed to achieve glycemic goals on maximal tolerated metformin dose, a second agent should be added. Options include SGLT2i, GLP-1 RA, sulfonylureas, DPP4 inhibitors, alpha-glucosidase inhibitors, thiazolidinediones and insulin. The choice of a second agent should be tailored to the individual patient.
Sodium-glucose cotransporter 2 (SGLT2) Inhibitors. This class works on the proximal renal tubules lowering the threshold for glucose excretion and increasing the urinary glucose clearance. This effect causes a light osmotic diuresis effect and net excretion of calories through the glucose urination.
A SGLT2 inhibitor is second tier in those with established atherosclerotic cardiovascular disease, those at high risk of heart failure or those with pre-existing heart failure or those with chronic kidney disease Multiple large randomized controlled trials namely CANVAS, CREDENCE, EMPA-REG OUTCOME and DECLARE-TIMI-58 have all shown that canagliflozin, empagliflozin and dapagliflozin resulted in reduction in heart failure and in DKD progression in patients with type 2 diabetes and established ASCVD or DKD. Empagliflozin statistically significantly decreased composite three-point major cardiovascular event: MI, stroke and cardiovascular death (MACE) outcome by 14% and cardiovascular deaths by 38% (EMPA-REG OUTCOME). Similarly, canagliflozen reduced occurrence of MACE in those at high risk for ASCVD vs placebo (CANVAS and CANVAS-Renal Trial). Dapagliflozin did not reach statistical significance for lower rates of MACE but significantly lowered cardiovascular deaths or hospitalization for heart failure (DECLARE-TIMI 58 and DAPA-HF trial).
In addition, these trials examined kidney effects. They indicated that they reduced the risk of incidence or worsening nephropathy. CREDENCE trial evaluated the primary outcome for composite of end-stage kidney disease, doubling of serum creatinine or death form renal or cardiovascular causes with canagliflozin 100mg vs placebo. Primary composite outcome was 30% lower with canagliflozin vs placebo. Overall, the endpoint of end-stage renal disease was reduced by 32% in the canagliflozin group. It is therefore, useful in patients at high risk of DKD progression (ie, with albuminuria or a history of eGFR loss). Prior to these studies, FDA strengthened the existing warning about the risk of acute kidney injury with canagliflozin and dapagliflozin. Providers should consider factors that may increase the risk of acute kidney injury prior to initiation. These factors include decreased blood volume, chronic kidney insufficiency, congestive heart failure, and taking other medications such as diuretics, blood pressure medicines called angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), and nonsteroidal anti-inflammatory drugs (NSAIDs). Check electrolytes and kidney function before and again 8 weeks after initiation of SGLT2 inhibitors.
Hypoglycemia is rare when used as monotherapy. There are recommendations to dose reduce insulin or other concomitant insulin secretagogues. Although not indicated for hypertension or obesity, this class can cause hypotension and slight weight loss (~400 kcal/day, but only 2.5% weight loss in one trial at 52 weeks suggesting a compensatory mechanism). Monitor for hypotension and adjust blood pressure medications as needed.
Although there are renal and cardiac benefits, there are adverse side effects and warnings that should be taken into consideration before prescribing.
- Studies show an increased risk for urinary tract infections as well as genital mycosis infections in users as the most common side effects. FDA released a warning of rare but serious cases of Fournier’s gangrene.
- In patients taking canagliflozin, risk of bone fracture increased along with decreased bone mineral density at the hip and lower spine, suggesting avoiding use in patients with history of osteoporosis.
- Two large trials concluded that canagliflozin increased risk of leg and foot amputations vs placebo (6.3 vs 3.4 participants per 1,000 patients-years; HR 1.97 [95% CI 1.41-2.75]).48,49 Consider factors that may put patients at risk for amputations including history of prior amputation, peripheral vascular disease, neuropathy and diabetic foot ulcers.
- Among users of dapagliflozin risk of bladder cancer increased in clinical trials suggesting avoiding use in patients with a history of bladder cancer.
- Trials have shown a mean transient acute reduction in GFR of about 4 points during the first 2 weeks followed by a progressive recovery and stabilization of renal function. After 12 months average decline in GFR becomes more favorable in the SGLT2i group than in the placebo group. No SGLT2i is currently approved for GFR <30.50
The FDA has issued a warning that SGLT2 inhibitors can cause to ketoacidosis in euglycemic patients. Patients on SGLT2i who become ill with dehydration, nausea, vomiting, or malaise are at risk especially after a decrease in insulin dose or with alcohol consumption. If glucosuria is significantly elevated this should prompt a work-up for ketoacidosis even in the absence of significantly elevated serum glucose levels. Discontinuation of the SGLT2i, hydration and increased carbohydrate intake along with an increase in insulin dose will allow the ketoacidosis to resolve in most patients with Type 2 Diabetes.51 While euglycemic ketoacidosis can occur in patients with Type 2 diabetes on SGLT2i, it is more common and more severe in patients with Type 1 diabetes on SGLT2i. Consider screening for insulin deficiency with C-peptide, glucose and anti-GAD antibody after the acute DKA episode has resolved.
GLP-1 RA (incretin mimetic agents). Exenatide (Byetta), Liraglutide (Victoza), Extended-Release Exenatide (Bydureon), Dulaglutide (Trulicity), Semaglutide injectable (Ozempic), Lixisenatide (Adlyxin) (see Table 6, injectable agents), Semaglutide oral (Rybelsus) are approved for type 2 diabetes. They are typically used with metformin or other oral agents. They enhance insulin release in presence of hyperglycemia, slow gastric emptying and suppress appetite, which can lead to weight loss in overweight individuals.
GLP-1 receptor agonists reduce risk of DKD progression and CVD events and therefore should be considered for patients with type 2 diabetes and DKD or ASCVD as second tier agents who require an additional agent to metformin to attain target or cannot use or tolerate metformin. Data, specifically LEADER trial, suggests that liraglutide demonstrate favorable renal effects and greater benefit for reduction of ASCVD. In people with type 2 diabetes with ASCVD or increased risk for ASCVD, the addition of liraglutide decreased MACE and mortality. Dulaglutide also demonstrated cardiac and renal benefits in the REWIND trial. There was a reduction in risk for nonfatal myocardial infarction, nonfatal stroke and CV deaths by 12% compared with placebo in the REWIND study. In SUSTAIN-6 trial, Semaglutide also demonstrated favorable effects on cardiovascular endpoints and composite indices for DKD. In PIONEER-6, oral semaglutide demonstrated non-inferiority versus placebo for major cardiovascular events indicating cardiovascular safety. For secondary outcome, it was strongly associated with reduction in CV and all-cause mortality. In the EXSCEL trial, MACE events were lower with extended-release exenatide vs placebo but it was not statistically significant. All-cause mortality was statically significantly lower in the extended-release extenatide group.
Oral semaglutide is the first GLP-1 RA administered orally and has similar efficacy compared to the injectable GLP-1 RAs. In studies, it was superior to empagliflozin and sitagliptin for A1c and weight reduction. Rybelsus has specific administration instructions. It should be taken at least 30 minutes before the first food, oral medication or beverages of the day, with no more than 4 ounces of plain water. It does not have any known drug-drug interactions but food may decrease the absorption of the drug.
Hypoglycemia is rare when these agents are used as a single agent or in combination therapy with metformin. The most common side effects are nausea and vomiting. The FDA warns that exenatide may be associated with an increased risk for pancreatitis and subsequent acute renal failure. If pancreatitis is suspected, incretin mimetic agents should be discontinued. If pancreatitis is confirmed, exenatide should not be restarted unless an alternative etiology for the pancreatitis is identified. Exenatide should not be used in those with GFR<30. It should be used cautiously in those with GFR between 30 and 50, with careful monitoring of renal function and GI side effects. Liraglutide, Dulaglutide and Semaglutide may be used with care in renal insufficiency. In large randomized control safety and efficacy trials for each individual medication, weight loss was seen in order of most to least in semaglutide, liraglutide, dulaglutide, exentaide and lastly lixisenatide.
Sulfonylureas. Sulfonylureas lower serum glucose by increasing insulin secretion. While sulfonylureas are no longer considered 1st line agents, in type 2 diabetes, they should be considered when cost is an issue or all other options are not tolerated or the patient does not have specific comorbidities. Compared to metformin, DPP4 inhibitors, SGLT-2i, GLP-1 RAs, sulfonylureas have less favorable effects on weight and increased risk of hypoglycemia. There has been debate of sulfonylurea and cardiovascular risk with weak evidence indicating that patients treated with sulfonylureas (mainly first generation) have higher cardiovascular mortality compared to patients treated with metformin. More recent large randomized controlled trials have suggested the lack of excess cardiovascular risk with glimepiride versus linagliptin and therefore suggests a neutral effect for glimepiride.
Glyburide, glipizide and glimepiride all have comparable efficacy at A1c reduction. For patients with any renal impairment, glipizide is preferred. Severe hypoglycemia can occur in patients with significant renal impairment. Avoid use of glyburide and glimepiride in elderly patients (>65 years) due to risk of prolonged hypoglycemia.
Patients are typically treated with a second-generation sulfonylurea starting at a low dose. Dose increments may be made every two weeks. If the patient has not achieved glycemic goal after four weeks of therapy at a maximal sulfonylurea dose, sulfonylurea therapy should be considered inadequate.
Non-sulfonylurea insulin secretogogues. These medications also lower serum glucose by increasing insulin secretion. They are often used in the place of sulfonylureas in sulfonylurea -allergic patients or when their shorter half-life and frequent dosing might reduce the risk of hypoglycemia in the event of skipped or delayed meals. Effects on weight and hypoglycemia risk are comparable to sulfonylureas.
Dipeptidyl peptidase-4 (DPP-4) inhibitors. Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are incretin hormones that stimulate insulin secretion and suppress glucagon. These incretin hormones are rapidly degraded by DPP-4. DPP-4 inhibitors enhance the effect of these incretin hormones by inhibiting DPP-4. A DPP-4 inhibitor may be used as monotherapy in the event of intolerance to metformin and is a useful second tier agent for use in combination therapy. DPP-4 inhibitors are not associated with weight gain. When used as monotherapy, hypoglycemia is rare with these agents. Data on the effects of these drugs on lipid profiles or cardiovascular outcomes is limited. Recent large randomized control trial indicated neutral effect on cardiovascular risk with linagliptin vs placebo.52 Dosage adjustments are required for renal insufficiency with sitagliptin, saxagliptin, and alogliptin but not with linagliptin. FDA safety review has found that saxagliptin and aloglitptin may increase the risk of heart failure, particularly in patients who already have heart or kidney disease. However, the EXAMINE trial showed no increase of cardiovascular events including heart failure with alogliptin compared to placebo, so the ADA recommends to avoid only saxagliptin in patients with heart failure.
Alpha-glucosidase inhibitors. Alpha-glucosidase inhibitors slow the digestion of ingested carbohydrates, delay glucose absorption into the bloodstream, and decrease postprandial blood glucose levels. Their effect on lowering A1c is minimal and there is no significant cardiovascular benefit with regards to mortality and morbidity, but they are safe in patients with heart disease.53 They are not associated with weight gain, nor do they cause hypoglycemia when used as monotherapy or in combination with metformin. Gastrointestinal side effects including abdominal pain, flatulence, and diarrhea are common. These effects usually diminish over time (4-8 weeks), but frequently lead to discontinuation of the drug.
Thiazolidinediones. Thiazolidinediones (TZD) reduce insulin resistance and lower blood glucose levels by improving sensitivity to insulin in muscle and adipose tissue. They reduce both glucose and insulin levels and do not cause hypoglycemia when used as single agents (or in combination with metformin). These medications are very effective at lowering A1c and have been shown to improve fibrosis in NASH, however due to their side effect profile, they should be considered only if cost or hypoglycemia is an issue. TZDs are associated with significant weight gain.
The FDA has issued a box warning for TZDs due to an increased risk of congestive heart failure (CHF). Therefore, these drugs should be avoided in patients with CHF. Both TZDs are associated with fluid retention and peripheral edema, which occur in at least 15% of patients. TZDs are strongly associated with increased fracture risk in post-menopausal women. TZDs may worsen diabetic macular edema. Renal dosage adjustment is not necessary. Pioglitazone has been associated with an increased risk of bladder cancer. FDA safety review recommends not to use in patients with active bladder cancer or history of bladder cancer.
Combination therapy. Since Type 2 diabetes is a progressive disease state, monotherapy treatment is often possible for only a few years in which combination therapy is often necessary thereafter. Patients with type 2 diabetes who do not have adequate glucose control on metformin will need to start an additional medication. (Figure 1) Studies like the VERIFY trial demonstrate that initial combination therapy had some benefits in extending therapeutic failure and slowing decline of glycemic control compared to initiating metformin alone. ADA recommends consideration of initial combination therapy in patients with A1c levels 1.5%-2.0% above target A1c goal. Therefore, treatment intensification for patients not meeting goal should not be delayed. The choice of medications in combination with metformin should be based on patient characteristics. In trials comparing addition of GLP-1 RA and insulin, the efficacy was similar with less hypoglycemia and more weight loss with GLP-1 RA. More gastrointestinal side effects were noted in patients taking GLP-1. It is preferable to add GLP-1 RA (with demonstrated CVD benefits), or a SGLT2i before starting insulin (if available and not contraindicated). This is especially true, for patients with established ASCVD or indicators of high ASCVD risk, heart failure, or DKD. When considering medication combinations, DPP-4 inhibitors should not be combined with GLP-1RA as it is considered a duplicative therapeutic. Most experts agree metformin should be continued if insulin is initiated. While it is common to discontinue hypoglycemic agents other than metformin when initiating insulin, it is not required. The weight loss and renal and cardio-protective benefits of SGLT2i and GLP-1A, as well as the benefits of reduced exogenous insulin dose, (lower cost, less weight gain) should be considered before discontinuing these agents.54,55
The addition of basal insulin including NPH, or one of the long-acting insulins, to oral medications is a common approach especially for those who have had diabetes for a longer duration of time. Once daily glargine therapy has become increasingly popular due to its convenience, lack of an insulin peak, and 24-hour duration of action. However, because long-acting insulin effects on glycemia are relatively constant throughout the day, this approach may make it difficult to address both nocturnal hypoglycemia and inadequately controlled post-prandial hyperglycemia simultaneously. Therapy may be intensified as needed with twice daily split/mixed insulin, or a basal/bolus insulin approach as needed to achieve glycemic goals.
If A1c is still above target and basal insulin has been titrated to a dose that is >0.5 units/kg/day or acceptable fasting blood glucose level, then consider addition of a GLP-1 RA or oral agents such as SGLT-2 inhibitors to minimize weight gain and potentially reduce the amount of insulin needed. Further intensification with prandial insulin can be done if more post prandial glucose lowering is needed for a patient with irregular eating and there is more needed for flexibility.
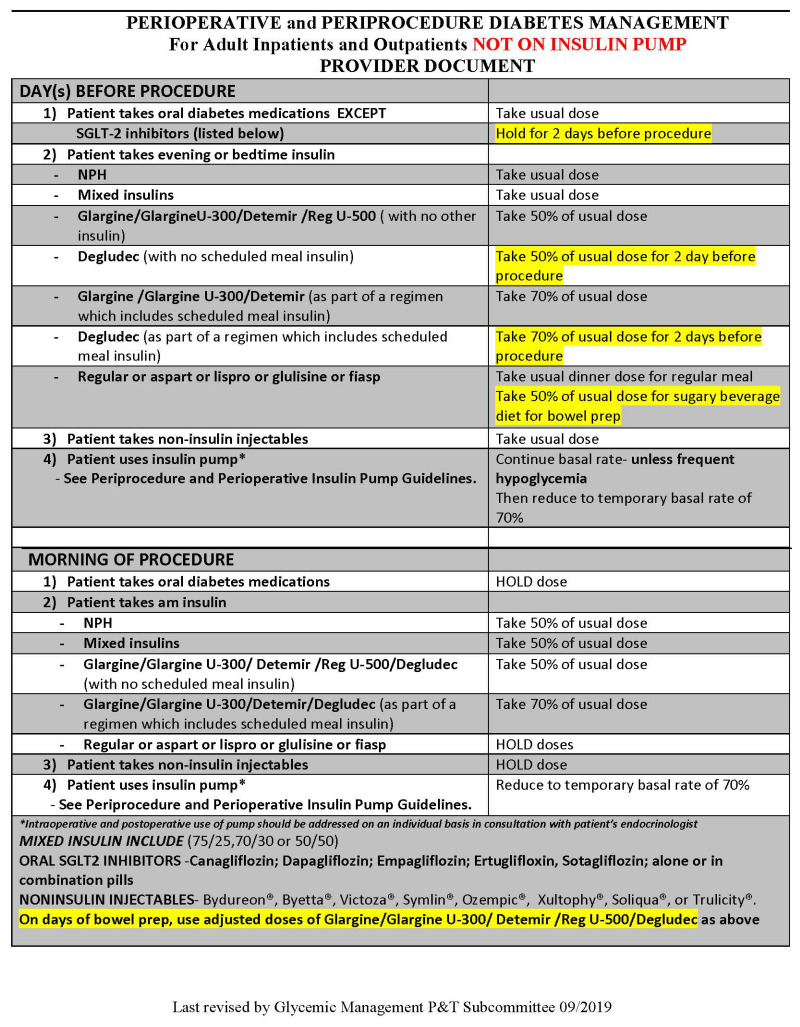
Insulin. Insulins are categorized by their duration of action (see Table 6). The initiation and adjustment of insulin is addressed in Appendix B.
Rapid acting insulins (Lispro [Humalog, Admelog], Aspart [NovoLog], Glulisine [Apidra]) or short-acting insulin (Regular) are used in conjunction with meals to treat anticipated post-prandial increases in blood glucose. Since the onset and duration of rapid-acting insulins are more physiologic than regular insulin, some practitioners prefer their use. However, in type 2 patients, regular insulin is an appropriate choice and is less expensive.
Intermediate insulins (NPH and Detemir [Levemir]) are typically given twice daily. A morning dose provides for daytime basal insulin requirements, and the post-lunchtime peak of action may reduce the need for short-acting insulin at lunchtime. An evening dose, often given at bedtime, is titrated to fasting blood glucoses, to avoid nocturnal hypoglycemia.
Long acting insulin, Glargine (Lantus, Basaglar, Toujeo) has a duration of action of approximately 24 hours. It is frequently prescribed at a starting dose of 20 units at bedtime in patients with normal renal function and titrated by 2 to 4 units every 2-3 days for fasting blood sugar <130 mg/dL. Ultra-long acting insulin, (Degludec [Tresiba]), may result in a lower hypoglycemia risk compared with U-100 glargine.
Mixtures of intermediate and short acting insulins are available in many forms. The three mixtures most frequently used are 75/25 insulin lispro protamine and insulin lispro (Humalog mix) and 70/30 insulin aspart protamine and insulin aspart (Novolog mix) and 70/30 NPH/insulin regular (HumuLIN 70/30, NovoLIN 70/30). Twice daily injections (before breakfast and supper) of these mixtures may provide good control for patients with type 2 diabetes. However, there is higher risk of hypoglycemia; therefore, education of not skipping meals is recommended to avoid hypoglycemia.
NPH and insulin regular are available as cheaper alternatives at pharmacies through discount programs.
All types of insulin are renally cleared and thus individuals with worsening chronic kidney disease or acute kidney injury will need to reduce their insulin dose to avoid hypoglycemia.
Medications that increase blood sugar
Hyperglycemia is clinically defined as glucose greater than 180 mg/dL for more than two hours. Medications commonly contributing to elevated blood glucose or hyperglycemia include atypical antipsychotics, corticosteroids, calcineurin inhibitors (cyclosporine, sirolimus, tacrolmus), and protease inhibitors. Corticosteroids are a very common cause of hyperglycemia in patients with or without diabetes because they blunt the action of insulin and promote hepatic gluconeogenesis. Clore and Thurby-Hay recommend using NPH insulin for treating glucocorticoid-induced hyperglycemia or basal/prandial insulin can be used as well.
For atypical antipsychotics, second generation antipsychotics may increase risk of hyperglycemia or type 2 diabetes – particularly olanzapine and clozapine whereas ziprasidone (Geodon) and aripirazole (Abilify) have the lowest risk. Newer atypical antipsychotics such as aspenapine, iloperidone, paliperidone and lurasidone also have lower metabolic risk similar to aripiprazole and ziprasidone.
Calcineurin inhibitors are often used to avoid allograft rejection in transplant which can result in post-transplant diabetes. The incidence of post-transplants diabetes is ~24% within 3 years post-transplant. These medications inhibit pancreatic islet beta cell expansion promoted by calcineurin.
Protease inhibitors are essential in the treatment of HIV and AIDS. They are thought to cause decrease in insulin sensitivity thereby resulting in insulin-resistance associated hyperglycemia. This occurs in 3-17% of patients.
Other agents that increase blood glucose include antibiotics specifically fluroquinolones (Gatifloxacin and Levofloxacin), beta blockers, statins, and thiazide diuretics. Beta blockers like atenolol, metoprolol and propranolol elevate blood glucose level by impairing release of insulin from pancreatic beta-cells. Carvedilol and nebivolol have not been associated with development of hyperglycemia or new-onset diabetes. For thiazides, like hydrochlorothiazide patients may not experience elevated levels for weeks (or not at all) if doses are kept low (12.5-25mg). Lastly, for statins, FDA drug safety announcement reported an increase in blood glucose and risk of diabetes with statins (JUPITER and PROVE-IT TIMI 22 trial) but that cardiovascular benefits outweighed risk of not using statins in patients with diabetes.
Vigilant monitoring of blood glucose should be done for patients on these medications irrespective of previous diabetes diagnosis. Use of these drugs are not contraindicated and clinical judgement while evaluating benefits versus risk of the use of these medications is recommended.
Hypoglycemia
Recommendations
Assess for hypoglycemia at every encounter in patients taking medication that increase the risk of hypoglycemia (especially insulin and sulfonylureas).
Assess patient for hypoglycemia unawareness.
- Modify medication, diet, and/or glycemic targets if patient has hypoglycemia unawareness, frequent or severe events requiring assistance for treatment.
- Consider using a continuous glucose monitor to detect and alarm for dangerously low glucose.
- Educate that beta-blockers can alter or inhibit symptoms of hypoglycemia thus masking symptoms of hypolycemia except sweating.
Fast-acting glucose is preferred for conscious patients with level 1 hypoglycemia or blood glucose <70 mg/dL (3.9 mmol/L) who are taking medication that can cause hypoglycemia.
- After treatment of hypoglycemia, recommend snack or meal to prevent recurrence of hypoglycemia.
Prescribe glucagon for patients at risk of level 2 hypoglycemia or glucose <54 mg/dL (3.0 mmol/L) or level 3 hypoglycemia defined as an event altering physical or mental status requiring assistance for treatment of hypoglycemia.
Educate patients on situations that increase risk for hypoglycemia, symptoms, and treatment of hypoglycemia.
Hypoglycemia is linked with increased risk of mortality thus outweighing the potential benefits on microvascular complications in some patients. It is also a marker of high absolute risk of cardiovascular events. Findings from ACCORD, ADVANCE and VADT trials caution aggressive approach in achieving glycemic targets in high-risk patients and recommends individualization of glycemic targets based on patient factors, comorbidity and life expectancy. Of note, these studies pre-date the GLP1-RA and SGLT2i drug classes where cardiovascular and renal benefits are seen.
Risk factors that increase treatment-associated hypoglycemia include: use of insulin or secretagogues, impaired kidney or hepatic function, longer duration of diabetes, frailty and elderly patients, declined cognitive function, hypoglycemia unawareness, alcohol use, polypharmacy (ie, beta blocker).
While healthy, non-diabetic individuals can be asymptomatic with fasting serum glucose as low as 45 mg/dL without concerns, in patients with Type 2 diabetes who are taking medications that increases the risk for hypoglycemia (especially insulin and sulfonylureas), the definition of hypoglycemia is glucose <70 mg/dL (3.9 mmol/L). Some patients with diabetes who desire very tight control (ie, pregnant women) may tolerate and possibly benefit from lower glucose levels without adverse effects, but the risk of serious hypoglycemia increases with tighter control. There are three levels of hypoglycemia. Level 1 is glucose <70 mg/dL (3.9 mmol/L) and ≥54 mg/dL (3.0 mmol/L). Level 2 is glucose <54 mg/dL (3.0 mmol/L) and level 3 is defined as a severe event marked by altered physical and/or mental status requiring assistance in the treatment of hypoglycemia. There are patients who have hypoglycemia unawareness which is defined as level 2 hypoglycemia without any symptoms.
Symptoms of hypoglycemia include but are not limited to: irritability, sweating, shakiness, confusion, lightheadedness, hunger, and dizziness. Level 3 hypoglycemia can result in seizures, loss of consciousness, coma, or death. A large trial suggested that a history of level 3 hypoglycemia was associated with greater risk of dementia. Symptoms may vary between patients; therefore, education and assessment of symptoms of hypoglycemia is important.
The best approach for treatment is fast-acting glucose or carbohydrate (15-20 grams) in patients who are conscious with blood glucose <70 mg/dL (3.9 mmol/L). Pure glucose is preferred versus the total carbohydrate content of the food as it results in quicker response. Any form of glucose is acceptable. Added fat and protein may blunt glucose and increase insulin response thus prolonging acute glucose response. Avoid carbohydrate sources high in protein or fat to treat hypoglycemia. Fifteen minutes after the glucose consumption, check glucose. If blood glucose is still <70 mg/dL (3.9 mmol/L), repeat with 15-20 grams of glucose. Once glucose returns to normal, then patient should have a meal or snack to prevent the recurrence of hypoglycemia.
Glucagon should be prescribed to patients who are at risk of level 2 and 3 hypoglycemia. Glucagon is indicated when patients are unable or unwilling to consume carbohydrates by mouth. Glucagon administration teaching should be given to caregivers, school staff, or family members.
Hypoglycemia unawareness, severe (one or more level 3 hypoglycemia) or frequent hypoglycemia is an indication for modification of treatment regimen and glycemic targets. In addition, consider prescribing a continuous glucose monitor to detect and alarm for dangerously low glucose. Beta blockers can inhibit symptoms of hypoglycemia such as tachycardia and flight or fight symptoms except sweating.
Assessment of cause of hypoglycemia and education on how to prevent and treat hypoglycemia is recommended. Patient education on situations (ie, lifestyle including diet and exercise) and medications that can increase risk of hypoglycemia should be provided.
Use of CGM technology can be a useful tool in reducing time in hypoglycemic range in people with hypoglycemia unawareness.
Co-Morbid Conditions
Hypertension
Hypertension (HTN) is the predominant predictor of adverse events in patients with type 2 diabetes. Treatment of blood pressure reduces risks of major cardiovascular events such as myocardial infarction, stroke, or cardiovascular death, and also reduces the risk of microvascular outcomes such as visual loss, photo-coagulation for retinopathy, and the development of end-stage renal disease. Treatment of HTN in patients with type 2 diabetes should be a high priority for clinicians.
The majority of patients with diabetes and HTN have essential hypertension. However, it is important to identify secondary causes of HTN such as renal artery stenosis, primary hyperaldosteronism, pheochromocytoma, Cushing’s disease, and oral contraceptive use in patients who remain refractory to therapy, or who have clinical syndromes suggestive of these conditions.
Blood pressure target. The target BP depends on the presence of other risk factors.
Without risk: 140/90 mmHg with no ASCVD, ASCVD 10-year risk < 10%, and no DKD. (ASCVD risk is based on the ACC/AHA pooled cohort ASCVD risk calculator. Diabetes is already considered in calculating ASCVD risk.).
With ASCVD, ASCVD 10-year risk ≥ 10%, or DKD:
- - <130/80 mmHg if without risk for hypotension (eg, without: orthostatic hypotension, heart failure, older age).
- - Consider <140/90 mmHg if risk for hypotension.
Both age and diabetes are important factors in the ACC/AHA ASCVD risk calculator, resulting in a BP target of <130/80 mmHG for most patients with diabetes. Having diabetes essentially doubles an individual’s risk that results from other factors. Even with normal values for blood pressure, cholesterol, and a history of no smoking, with diabetes men age ≥ 55 years and women age ≥ 65 years will have a 10 -year ASCVD risk >10%. Many middle-age adults and some younger adults with diabetes and with other risk factors for ASCVD will have a calculated 10-year ASCVD risk >10%.
For patients at risk for hypotension (eg, orthostatic hypotension, heart failure, older age), consider a treatment target of <140 mmHg systolic and <90 mmHg diastolic blood pressure. The BP target is higher to avoid hypotension, which may result in insufficient blood flow to organs (eg, kidneys in patients with DKD), dizziness, and fainting.
Clinical trial data reviewed by the Seventh Report of the Joint National Committee (JNC 7) support reducing SBP to <140 mmHg and DBP to <90 mmHg. This was confirmed by the panel members of the Eighth Joint National Committee for ages 60 years and younger. For ages 60 years and over, the latter recommended reducing SBP to <150 mmHg and DBP to <90 mmHg. The 2017 ACC/AHA guidelines recommended reducing to <130 mmHg systolic and to <80 mmHg diastolic, based on new data from SPRINT. Systolic blood pressure had not been evaluated as rigorously as diastolic until SPRINT looked at SBP control and clinical outcomes. For patients with elevated blood pressure and elevated ASCVD risk, aggressive treatment of HTN provides significant improvements in clinical outcomes. Recent data suggest that a SBP target of <130 mmHg is reasonable.
In all guidelines, accurate BP measurement using automated office BP measurements or home BP measurement was recommended. A sustained decrease in SBP of 10 mmHg or DBP of 5-6 mmHg for patients with hypertension decreases the risk of stroke by 35-40% and decreases the chance of coronary heart disease by 20-25%.
For patients with diabetes, goals for blood pressure treatment have been evaluated in several randomized trials, particularly ACCORD. SPRINT did not include patients with diabetes. For DBP, a target of ≤90 and likely ≤80 mmHg provides marked benefits. Caution is suggested when DBP falls below 70 mmHg. Mortality increased when patients with diabetes had DBP below 70.
The American Diabetes Association’s 2019 Standards for Medical Care in Diabetes synthesize results from ACCORD and SPRINT by focusing on diabetes as a risk factor for ASCVD. The ADA recommends that BP targets for patients with diabetes be based on the patient’s ASCVD status and 10-year risk for ASCVD, consistent with the ACC/AHA approach to setting BP targets based on ASCVD and ASCVD risk. The one difference is that for a BP target of <130/80 mmHg, ACC/AHA set 10-year ASCVD risk level at ≥10% and the ADA set the level at ≥15%. This difference is of little practical consequence. The effect of increasing age on the calculation of ASCVD risk is sufficiently strong than anyone with an estimated 10-year risk that is >10% and <15% will have an estimated risk ≥15% within a couple of years. Using 10-year ASCVD risk level of ≥10% initiates lowering the goal to <130/80 mmHg slightly earlier.
Blood pressure assessment and treatment. Blood pressure should be measured at all clinic visits for patients with diabetes, and treatment is more aggressive than for patients without diabetes. If diastolic blood pressure is ≥ 90 mmHg or systolic blood pressure is ≥ 140 mmHg on two visits, antihypertensive therapy should be instituted (Tables 6 and 9). Lifestyle modification with dietary alteration, physical activity, and weight loss (if indicated) should be advocated. However, expert opinion from The Seventh Report of the Joint National Committee on Detection, Evaluation and Treatment of High Blood Pressure (JNC VII) recommends that in patients with diabetes, lifestyle measures should nearly always be augmented by pharmacologic therapy.
Recommendations
Initiate pharmacologic agent addition to lifestyle modifications, if blood pressure in clinic is ≥140/90 mmHg.
Initiate two drugs or single-pill combination of drugs in addition to lifestyle modifications, if blood pressure in clinic is ≥160/100 mmHg to reduce cardiovascular events.
Treat hypertension with ACE inhibitors, angiotensin receptor blockers (ARB), thiazide-like diuretics or dihydropyridine calcium channel blockers as these drug classes have demonstrated reduction in cardiovascular events in patients with diabetes.
Use ACE inhibitors or ARBs are first line treatment for hypertension in patients with diabetes and CAD, or urinary albumin-to-creatinine ratio ≥300 mg/g creatinine or 30-299 mg/g creatinine
Monitor EGFR serum creatinine, and electrolytes (potassium) if using ACE inhibitor, ARBs or thiazides after initiation and then at least annually.
Do not use ACEI inhibitors in combination with ARBs.
Initial treatment is based on severity of hypertension. Patients with blood pressure ≥140/90 mmHg, a single agent can be initiated. Patients with blood pressure ≥160/90 mmHg, initial treatment should include a two drug therapy.
The choice of first-line antihypertensive drugs for patients with diabetes is controversial and not entirely based on the available literature. In the ALLHAT trial, the largest and most representative direct drug-vs.-drug comparison to date, a strategy beginning with a thiazide diuretic (chlorthalidone) reduced myocardial infarction as much as strategies beginning with other agents and reduced stroke and congestive heart failure more than beginning with other agents. That result held across all subgroups, including patients with diabetes. The ADA guidelines recommends Angiotensin-converting enzyme (ACE) inhibitors and Angiotensin Receptor Blockers (ARBs) as first-line therapy for hypertension in patients with established coronary artery disease and diabetes (HOPE trial).
ACE inhibitors and ARBs reduce progression of established diabetic renal disease and reduce cardiovascular mortality (HOPE trial). Thus, ACE inhibitors or ARBs are recommended as first-line therapy in patient with albuminuria (urine albumin-to-creatinine ratio [UACR] ≥30 mg/g). An important note is that the combination of ACE inhibitors and ARBs should be avoided. Although together they reduce blood pressure and proteinuria, they also clearly increase the rate of end-stage renal disease and mortality. In patients without albuminuria or kidney disease, ACE inhibitors and ARBs have not been found to be superior for cardioprotection when compared to thiazide-like diuretics and dihydropyridine calcium channel blockers.
Dihydropyridine calcium-channel blockers was superior in reducing cardiovascular events when in combination with a renin-angiotensin system blocker (benazepril) compared to hydrochlorothiazide in patients with diabetes (ACCOMPLISH trial).
Beta-blockers are also effective agents in controlling blood pressure, but should probably be added after thiazides and ACE inhibitors, ARB or dihydropyridine calcium channel blockers (see Table 8). Beta blockers are beneficial for treatment of prior MI, active angina, or heart failure but have not been shown to reduce mortality in absence of these disease states. In patients taking insulin for Type 2 diabetes, beta blockers can worsen hypoglycemia episodes particularly during acute illness or hospitalization.56 Other classes of agents have not been as rigorously evaluated in patients with diabetes. Alpha-blockers are not recommended, as they appear to deliver less improvement in outcome than other agents.
Low-dose thiazide diuretics (eg, 12.5 to 25 mg of hydrochlorothiazide or 25-50 mg chlorthalidone) do not appear to have clinically important adverse effects, and have been proven to reduce mortality in patients with diabetes. High-dose thiazide diuretics have been reported to have a variety of adverse effects including worsening of hyperlipidemia, hyperuricemia and gout flares, deterioration of glycemic control, impotence, and increased mortality, therefore thiazides should be used at low doses.
Patients with coronary disease or congestive heart failure (CHF) should receive beta-blockers unless a clear contraindication exists. Beta-blockers may decrease high density lipoprotein (HDL) and increase triglyceride levels. In one major trial, beta-blockers led to more weight gain and higher requirements for glucose-lowering agents than ACE inhibitors. If a beta-blocker is used, it should be cardioselective to minimize side-effects.
Patients with CHF or coronary disease with diminished left ventricular function should receive an ACE inhibitor, or an ARB (if ACE inhibitors are not tolerated). ACE inhibitors can lead to cough in up to 20% of patients. Both ACE inhibitors and ARBs can precipitate renal insufficiency and hyperkalemia. Careful monitoring of renal function and serum electrolytes is warranted with these agents.
Regardless of initial agent, most patients with type 2 diabetes will require multiple agents in order to achieve their blood pressure goal. Indeed, many patients will not achieve their goal even with the use of 3 or 4 agents. Further evaluation for secondary causes of hypertension should be considered in these patients.
Lastly, there has been small evidence suggesting reductions in ASCVD events when dosing at least one antihypertensive medication in the evening vs morning.57
Lipid screening and treatment
Prescribe at least a moderate potency statin for patients with Type 2 diabetes who are ≥40 years old. Avoid statins in women who are contemplating pregnancy or may become pregnant.
Hyperlipidemia is common in patients with type 2 diabetes. Characteristically, they have elevated triglyceride levels, while HDL levels are low, and LDL levels are typically normal or elevated. Given the high prevalence (up to an 80% lifetime risk) of vascular disease in patients with diabetes, the National Cholesterol Education Program (NCEP) suggests that lipid-lowering treatment is an essential component of diabetes care.
Expert opinion suggests that annual lipid profile provides a check on statin adherence and an opportunity to reinforce lifestyle modifications – the cornerstone of ASCVD risk reduction.
A non-fasting lipid profile is adequate to assess cardiovascular risk and to monitor statin compliance. If lipids are obtained non-fasting and are abnormal (ie, TC >200 mg/dL, HDL-C <40 mg/dL, or triglycerides >500 mg/dL,) consider obtaining a follow up fasting lipid panel to better evaluate for dyslipidemias.
Current ADA and AHA guidelines recommend that for patients with diabetes and known ASCVD or ASCVD risk greater than 20%, treatment should be initiated with high dose statin. If LDL <70 mg/dL or non-HDL cholesterol ≥100 mg/dl is not achieved on maximally tolerated statin therapy, addition of a second agent, like ezetimibe or a PCSK9, should be considered. For patients with diabetes who are <40yo, a statin should be started if LDL ≥190 mg/dL with goal of achieving a 50% reduction.
For patients with diabetes under the age of 40, and without extreme elevations of LDL, a moderate dose statin could be considered if there are multiple ASCVD risk factors.
For patients with diabetes 40-75 years old without known ASCVD and an ASCVD risk <20%, at least a moderate potency statin should be started. For patients with diabetes >75 years without known ASCVD and an ASCVD risk <20%, any previously prescribed statin should be continued. Consider a moderate dose statin for patients with diabetes age >75 years without known ASCVD after a risk/benefit discussion.
The issue of LDL targets is controversial. Experts have suggested LDL targets of less than 100 or even 70 mg/dL for patients with diabetes. However, few studies have established a specific LDL target level; instead nearly all trials compared the efficacy of a fixed dose of a statin with placebo. The best evidence suggests that patients receive about the same level of benefit across all baseline LDL levels and with any degree of LDL reduction. This suggests that the benefits of statins are not fully captured by LDL and argues for their empiric use. A reasonable approach is to start most patients with diabetes on moderate potency statins, (eg, lovastatin [generic] 40 mg/dL) without specific LDL targets. For secondary prevention, essentially all patients with diabetes should be on statins; some evidence supports the use of higher dose statins in these populations (eg, rosuvastatin 40 mg/dL or atorvastatin 40-80 mg/dL), particularly in those who are admitted for acute coronary syndrome. Avoid prescribing simvastatin 80 mg because of the increased risk of myalgias. Careful monitoring of potential drug interactions with statins is critical; many drugs can increase the risk of myalgias and rhabdomyolysis when combined with statins.
Non-HDL is a measurement of all atherogenic lipoproteins (lipoprotein(a), very low-density lipoproteins (VLDL), intermediate density lipoproteins (IDL), and low-density lipoproteins (LDL)). Unlike LDL, non-HDL is not dependent on fasting and is more accurate when triglycerides are elevated. Non-HDL thresholds for adults should be 30 mg/dL higher than the equivalent LDL threshold.
Statins may not be appropriate in some patients with diabetes, especially those with severe, chronic malnutrition from pancreatic insufficiency, or women planning pregnancy. When deciding to start a statin, consider the patient’s 10 year ASCVD risk, nutritional status and life expectancy.
In patients with diabetes, observational data suggest that triglycerides are also an independent risk factor for the development of atherosclerotic disease. However, only very limited trial data evaluate the effectiveness of lowering triglycerides on cardiovascular outcomes. The first-line of treatment for hypertriglyceridemia is optimization of glucose and thyroid (if hypothyroid) control. Use of fibrates is generally discouraged as there is no evidence of benefit in trials using fibrates alone or in combination with statins. If triglycerides are markedly elevated (eg, >500 mg/dL), then treatment may be warranted to avoid pancreatitis.
Non-alcoholic fatty liver disease (NAFLD)
It is estimated that approximately 25-30% of adults in the United States have underlying NAFLD.58 NAFLD has been reported in one- to two-thirds of adult patients with type 2 diabetes.59,60 Despite this high prevalence, there is some debate about routine screening in high-risk patient groups “because of uncertainties surrounding diagnostic tests and treatment options, along with lack of knowledge related to long-term benefits and cost-effectiveness of screening”.61 It is recommended that clinicians maintain a high index of suspicion for NAFLD and Non Alcoholic Steatohepatitis (NASH) in patients with type 2 diabetes.61 In the 2021 ADA guidelines, patients with type 2 diabetes who have elevated transaminases or evidence of fatty liver on ultrasound should be evaluated for NASH and fibrosis.62 Non-invasive risk-stratification tools may be helpful to identify individuals at low or high risk of advanced fibrosis. These include serologic biomarker calculators like the NAFLD fibrosis score (NFS), the fibrosis-4 index (FIB-4) or vibration controlled transient elastography (VCTE).61 Non-alcoholic steatohepatitis (NASH) is a subgroup of people with NAFLD who have inflammation in addition to fatty infiltration and are thus at high risk of progression to cirrhosis. NASH is currently one of the leading indications for liver transplant in the US. The only way to definitively diagnose NASH is by liver biopsy. The primary treatment that slows the progression of NAFLD/NASH is diet, exercise, and weight loss with strong evidence for benefit from adopting a Mediterranean diet or a low carbohydrate diet.7,8 Additional benefits may come from decreasing or eliminating alcohol consumption, vaccinating against hepatitis A and B, screening for hepatitis C, and treating if appropriate, avoiding liver toxic medications, supplements, and alcohol. Preliminary data supports the use of SGLT-2 inhibitors and GLP-1 agonists and larger trials of these medications are ongoing.10,63 Individuals identified as being high risk for advanced fibrosis, or individuals with indeterminate risk assessment scores and multiple metabolic comorbidities, may benefit from referral to GI/Hepatology.
Obesity
Obesity is increasing worldwide and contributes to the rise of not only type 2 diabetes, but also hypertension, hyperlipidemia, macrovascular disease, osteoarthritis, and other conditions. The treatment of obesity is central to the comprehensive treatment of type 2 diabetes in many cases. Lifestyle interventions for obesity, medications to promote weight loss, and bariatric surgery should all be considered in the approach to the obese patient with type 2 diabetes.
The majority of patients with type 2 diabetes have overweight or obesity. Weight loss of 5-10% can help patients with type 2 diabetes reduce or eliminate the need for anti-hyperglycemic medications.13,64 A comprehensive weight management approach that considers individual patients’ barriers to weight loss as well as their needs, preferences, and goals is recommended.
Addressing barriers:
- Replace obesogenic medications with weight-neutral alternatives, if appropriate.
- Screen for medical conditions (eg, obstructive sleep apnea, insomnia, Cushing syndrome, hypogonadism, hypothyroidism, polycystic ovarian syndrome, depression, or eating disorders) or life events (eg, marriage, divorce, retirement) that may promote weight gain and/or hinder weight loss.
- Ask about access to food and refer to Social Work for food insecurity resources, if appropriate
Developing a personalized weight loss plan:
Primary care-based resources: Although Medicine reimburses PCPs for intensive lifestyle counseling (ILC), most PCPs do not have the time or knowledge to engage patients in ILC.65 Accordingly, PCPs should consider referring patients with obesity to dietitians, clinical pharmacists and/or evidence-based lifestyle change programs.
Lifestyle change programs: Lifestyle programs aim to help patients lose weight through diet and physical activity changes. Patients should be educated that dietary changes are key to weight loss (see Nutrition section). Exercise promotes cardiovascular and mental health benefits, but is not required for weight loss. Exercise does, however, play a key role in weight loss maintenance.66 Commercial lifestyle change and meal replacement programs supported by the literature include: Weight Watchers, Jenny Craig, Nutrisystem, Medifast, Optifast, Atkins, Slim-fast, TOPS and Health Management Resources.66–68 Vitra Health’s online keto diet program for people with type 2 diabetes has been shown to induce diabetes remission in some type 2 diabetes patients.69
Pharmacotherapy: May be considered in patients who have failed to reach weight loss goals through lifestyle modification alone, have obesity-related health issues, and have BMI >30 kg/m2; or BMI >27 and at least one obesity-related health condition. A list of potentially obesogenic medications and non-obesogenic alternatives is listed in Table 11. Table 12 lists medications for weight loss.
Table 11
Obesogenic medications and alternatives for common conditions ,.
Table 12
Medications for Weight Loss ,,.
Supervised medical weight loss: Often includes very low energy meal replacement (<800 kcal per day).
Weight loss surgery: Gastric bypass, laproscopic gastric banding, sleeve gastrectomy, or biliopancreatic diversion with duodenal switch may be considered in patients with BMI ≥40; or BMI ≥35 and suffer from an obesity-related condition.70,71 Consider a patient who is prepared and willing to commit to lifestyle changes that will be necessary after surgery eg, non-smoker; failed to lose weight with other approaches. These are associated with improvement or resolution of obesity-related chronic conditions and reduced mortality.72,73
Regardless of the treatment use, weight regain can occur. Strategies to promote weight loss maintenance (ie, maintain body fat mass within +5%) include regular physical activity, continued dietary adherence, self-weighing, and maintaining food log.74
Psychological comorbidities
- Psychosocial care should be integrated into a collaborative patient-centered approach and provided to all people with diabetes.
- Consider assessing depression, diabetes distress, anxiety, eating disorders, and cognitive capacities using validated tools at the initial visit, at periodic intervals, and when there is a significant change in health, treatment, or major life circumstances. Caregivers and family members should be included when this is appropriate.
Pain
Recommendation
Recommend low impact exercise such as to yoga, tai chi, and warm pool-based activities to help improve quality of life for patients with chronic musculoskeletal pain.
In addition to causing diabetic peripheral neuropathy, diabetes predisposes to chronic musculoskeletal pain. Observational studies have shown an increased risk for chronic back, shoulder, and neck pain. Diabetes appears to increase disability and emergency room visits due to chronic pain. Chronic pain may be a barrier to achieving diabetes-related benchmarks. Chronic pain presents a major barrier to frequent exercise, which is a key part of diabetes management. Low impact activities such as yoga, tai chi, and warm pool-based exercise have all been shown to improve quality of life and some diabetes-related outcomes.
Macrovascular Disease
Diabetes increases an individual’s risk of coronary artery disease, stroke, and peripheral vascular disease. Reducing other cardiovascular risk factors (Table 9) in patients with diabetes reduces their overall risk. Cardiovascular risk factors should be assessed annually in patients with type 2 diabetes. These risk factors include hyperlipidemia, hypertension, smoking, a family history of premature coronary disease, obesity, and the presence of micro- or macroalbuminuria.
Aspirin
Recommendations
Prescribe aspirin for secondary prevention to patients with a history of atherosclerotic cardiovascular disease.
Most patients with Type 2 diabetes and no ASCVD history do not benefit from aspirin for primary CVD prevention.
The ADA and most other organizations recommend use of aspirin for secondary prevention in all patients with diabetes who have known atherosclerotic cardiovascular disease. However, the use of aspirin for primary prevention of atherosclerotic disease events is not recommended form most people with diabetes as a routine practice. Recent data do not consistently show that aspirin used for primary prevention leads to a decrease in major cardiovascular events. Aspirin used for primary prevention increases the occurrence of major bleeding events such as GI bleeding and intracranial hemorrhage due to falls.75–78 This is an area of ongoing debate and research. The USPSTF states that there is some evidence to support using aspirin for primary prevention for patients between the ages of 50 and 69 who are at high risk for adverse CVD events and at low risk for GI bleeding.79
SGLT2 inhibitors and GLP-1 RAs
Recommendation
Consider initiating an SGLT-2 inhibitor or GLP1-RA for patients with a history of ASCVD or those at high risk for ASCVD.
Multiple randomized controlled trials have demonstrated the cardiovascular benefit of these drug classes in people with type 2 diabetes.80–85 A reduction in major adverse cardiovascular events (MACE) and in some instances a reduction in mortality have been demonstrated for members of both these drug classes. These findings have led the ADA to recommend initiation of these drugs for diabetic patients with history of ASCVD or those at high risk for ASCVD. The term “high risk” has been variably defined, but generally includes individuals without history of ASCVD but presence of classical CVD risk factors such as hypertension, LDL >140, LVH, and tobacco smoking. Review the Glycemic Management section for a more information on the efficacy of the SGLT-2 inhibitors and GLP1-RAs.
Screening for Coronary Artery Disease
Recommendations
Do not screen for coronary artery disease in asymptomatic patients.
Consider screening for coronary artery disease in sedentary patients who plan to begin a vigorous exercise program.
Clinicians should maintain a high index of suspicion for macrovascular disease in patients with type 2 diabetes. Symptoms suggestive of coronary artery disease, transient ischemic attack, stroke, or peripheral vascular disease should prompt consideration of further testing.
Screening for coronary artery disease in asymptomatic individuals is not recommended as a routine practice. A large randomized control trial demonstrated that screening for CAD in asymptomatic type 2 diabetes did not reduce the rate of cardiac death or myocardial infarction.86
However, screening for coronary artery disease may be considered for individuals over age 30 with type 2 diabetes and additional risk factors for CVD who wish to start an exercise program more rigorous than a brisk walk. The prospective Look AHEAD study showed 22% of people with diabetes who were asymptomatic of coronary artery disease displayed objective abnormalities on exercise ECG stress testing.87
Cardiovascular Autonomic Neuropathy
Cardiovascular autonomic neuropathy (CAN) is defined as the impairment of autonomic control of the cardiovascular system. CAN is particularly concerning because it may lead to silent ischemia and/or silent myocardial infarction. CAN may manifest clinically as resting tachycardia, orthostatic hypotension, syncope, impaired blood pressure/heart rate response to exercise, and exaggerated drop in blood pressure/heart rate during induction of general anesthesia.
Autonomic neuropathy and cardiovascular disease
Although less common in Type 2 than Type 1 diabetes, autonomic neuropathy can occur. This is primarily of concern in the detection of cardiovascular disease, as angina may be silent in adults with diabetes. Care should be taken to elicit a history of possible atypical anginal symptoms or equivalents and consideration should be given to risk assessment and stress testing.88
Microvascular Complications
Include microvascular disease in screening and treatment (Table 7).
Retinopathy
Recommendations
Refer for a dilated retinal exam by an eye care specialist every 2 years if previous eye exam was normal and good glucose and BP control. Otherwise, annually or more frequently as recommended by the eye care provider.
Ophthalmologists should treat diabetic retinopathy.
Consider intensifying glycemic control and blood pressure control for patients diagnosed with diabetic retinopathy.
Consider more frequent dilated eye exams in patients being initiated on insulin, sulfonylureas, GLP1-RAs, and TZDs as they may increase risk for development of diabetic retinopathy.
Retinopathy and macular edema affect a substantial proportion of patients with Type 2 diabetes. Between 10 and 30% of subjects have retinopathy at the time of diabetes diagnosis, and most will eventually develop some level of retinopathy. Severe retinopathy requiring treatment is somewhat less common, but still makes diabetes the leading causes of visual loss in US adults and the leading cause of blindness in working age adults. Prevention of retinopathy is best achieved by optimizing blood pressure and glucose control.
Dilated retinal examination reduces the incidence of severe visual loss by allowing timely treatment (eg, laser photocoagulation, anti-VEGF intraocular injections) of proliferative retinopathy and macular edema. Optimal screening intervals for retinopathy depend on the risk in the individual patient. Patients who have been diagnosed with retinopathy should be screened at least annually, and many will require much more frequent examination depending on the degree of retinal abnormality. Patients have a low risk of developing retinopathy that will require treatment over the short term if they have no retinopathy on a baseline retinal exam by an expert and have both reasonable glucose and blood pressure control. These patients can be screened less frequently, at 2 year intervals. For measuring quality of care for diabetes, the HEDIS interval for retinal examinations is biennially for patients with previous normal eye exam and at least annually for patients with abnormal eye exam.
Unless the primary caregiver has been specifically trained to perform dilated retinal examinations, the accuracy of fundoscopic examination is poor. Thus, all screening should be performed by a trained eye-care professional.
Glucose-lowering drugs capable of producing rapid drops in A1c have been associated with increased rates of diabetic retinopathy. Insulin, sulfonylureas, TZDs, and GLP1-RAs have all been associated with increased rate of retinopathy presumable due to rapid reduction in glucose levels. If these medicines are being initiated, more frequent A1c checks and fundoscopic exams should be considered.
Nephropathy
Recommendations
Check spot urinary albumin/creatinine ratio and creatinine, electrolytes and eGFR annually.
If albumin/creatinine ratio >30 mg/gm, check UA to rule out asymptomatic UTI and repeat spot urine ratio twice within 6 months.
If 2 of 3 spot urine albumin/creatinine ratios >30 mg/gm:
- Begin ACE inhibitor or ARB and recheck creatinine and electrolytes within 1–2 weeks of initiating therapy.
- Consider initiating an SGLT2 inhibitor if A1c is above the patient’s individualized goal and the GFR is >30 mL/min.
Patients with diabetes with a glomerular filtration rate (GFR) <30-45 mL/min/1.73m2 with or without nephrotic range proteinuria should be referred to a nephrologist for evaluation for other causes of nephropathy and for discussion of potential treatment options.
Diabetic nephropathy affects 20%-40% of patients with diabetes and is the single leading cause of end-stage renal disease (ESRD) in the United States. A CDC analysis showed the age-adjusted incidence of ESRD caused by diabetes declined by one-third from 1996 to 2007, which may be related to more screening and aggressive use of ACE/ARB in treatment of blood pressure. Yearly screening and treatment for microalbuminuria can reduce the incidence of renal failure. The spot urinary albumin-creatinine ratio is a simple method for testing for microalbuminuria. Because of day-to-day variation in urinary albumin excretion, the test should be repeated on at least two more occasions over a 3- to 6-month period, if the first test result is positive. Two of three tests should be positive (>30 mg albumin per gm of creatinine) before microalbuminuria is considered present. Albuminuria is defined as albumin excretion >300mg/day.
Causes of elevated urinary albumin excretion in the absence of diabetic nephropathy include urinary tract infection, recent exercise, acute febrile illness, hematuria related to urinary tract infection (UTI) or menses, and congestive heart failure. If screening microalbumin is >30 mg/dL, check urinalysis to assess for other causes.
Microalbuminuria is a marker for greatly increased cardiovascular morbidity and mortality for patients with diabetes. Therefore, aggressive intervention is recommended to reduce all cardiovascular risk factors (eg, lowering of LDL cholesterol, antihypertensive therapy, cessation of smoking, institution of regular physical activity, etc.).
For people with diabetes and diabetic kidney disease (either micro- or macroalbuminuria), reducing the amount of dietary protein below usual intake is not recommended because it does not alter glycemic measures, cardiovascular risk measures or the course of GFR decline. Consider dietary referral to evaluate dietary protein in patients with proteinuria.
Multiple glucose lowering drugs have been shown to improve renal outcomes. A 2019 meta-analysis of RCTs that included patients with DKD and GFR>30 mL/min/1.73m2 showed SGLT2 inhibitors reduced the incidence of decline in GFR, need for renal replacement therapy, doubling of serum creatinine, and development of albuminuria.89 DPP4 inhibitors have been shown to decrease albuminuria. GLP1-RAs have been shown to reduce development of albuminuria. The evidence favors SGLT2 inhibitors, compared to other drug classes and therefore, should be considered for glycemic management in patients with DKD with a GFR of >30 mL/min, whose A1c is above goal. However, SGLT2 inhibitors have been associated with genitourinary tract infection, acute renal failure, and increased risk of diabetic foot amputation, therefore consider the risk factors carefully.
ACE inhibitors reduce the rate of progression from microalbuminuria to overt proteinuria and diabetic nephropathy, independent of their effect on blood pressure. ARBs show similar benefits to ACE inhibitors in patients with type 2 diabetes and microalbuminuria and diabetic nephropathy. Direct comparisons between ACE inhibitors and ARBs have not been performed in patients with type 2 diabetes. ACE inhibitors and ARBs are regarded as functionally equivalent in protecting against progressive diabetic nephropathy, although more evidence exists in the literature for therapy with an ARB to continue to show benefit even up to the development of end stage renal disease. An ACE inhibitor or an ARB should be used in all patients with microalbuminuria. Combination ACE/ARB therapy for patients with persistent albuminuria is NOT recommended. While the combination reduces proteinuria, it also increases renal failure and adverse events in patients with diabetes, without any benefits on cardiovascular or renal outcomes.
Other antihypertensives (including beta-blockers and non-dihydropyridine classes of calcium-channel blockers (NDCCB) can reduce the level of albuminuria, but no antihyptertensive studies to date have demonstrated a reduction in the rate of fall of GFR. Some members of the dihydropyridine class of calcium channel blockers (eg, nifedipine, felodipine) may increase urinary albumin excretion, and should be avoided in patients with microalbuminuria.
Control of blood pressure is important. Recommended blood pressure goals in patients with diabetes and chronic kidney disease are:
| Urine Albumin Excretion | Blood Pressure Goal |
|---|---|
| <30mg/24 hours | <140/90 (recommended) |
| >30mg/24 hours | <130/80 (suggested) |
In normotensive patients with microalbuminuria, target dosages of ACE inhibitors are difficult to define. Some experts recommend titrating medications upward until a normal albuminuria is seen or side effects occur.
For further information regarding care of patients with chronic kidney disease, see the UMHS clinical guideline on Chronic Kidney Disease.
Neuropathy
Diabetic neuropathy is reported in up to half of patients with diabetes. Most have loss of sensation, only a minority experience pain. Patients often describe pain as burning, shock sensation, or stabbing. Evidence indicates early detection of diabetic neuropathy and aggressive foot care results in fewer foot ulcers and amputations. Pay careful attention to the etiology of pain. Occasionally, mechanical factors rather than neuropathy are the mechanism underlying pain.
Glucose lowering medications may have an effect on neuropathy-related complications. GLP-1-RAs have been shown to decrease the risk of diabetic foot ulcer complications. Though SGLT-2 inhibitors on the whole have not been shown to increase the risk of diabetic foot ulcer complications, the CANVAS study results suggest that canagliflozin increases the risk of lower extremity amputations.48 Risk factors for lower limb amputation should always be considered prior to initiation of this drug class. Risk factors for lower limb amputations include significant peripheral neuropathy, peripheral arterial disease, diabetic foot ulcer, and prior amputation.
Diabetic foot care. Foot care includes examination, preventive care, consideration of orthotic footwear, and treatment of foot ulcers.
Examination. Perform a visual foot inspection, pulse and sensation check annually, and with every routine visit if abnormalities are present. Identify areas of callus formation, claw toe deformity, prominent metatarsal heads (or other bony prominences), and other structural changes. Three simple tests detect peripheral neuropathy: pressure sensation, vibration sensation and temperature/pain perception.
Perform sensory testing with a 5.07 (10g) nylon monofilament annually, to identify insensate feet without protective sensation. Instructions on “How to Use a Monofilament” are in Table 10. Individuals with insensitive feet are at high risk of developing foot ulcers and other related complications.
Table 10
How to Use a Monofilament to Test for Foot Neuropathy.
Education. All patients require education regarding optimal foot and nail care, which includes daily inspection and appropriately fitting shoes. To minimize the risk of trauma, patients should be counseled to avoid walking barefoot and those with neuropathy should avoid high-impact exercise and the use of hot water.
Footwear. Orthotic footwear should be prescribed to accommodate major foot deformities and off-load pressure areas. Most insurance plans, including Medicare, cover therapeutic footwear for patients with diabetic neuropathy or deformity. For others with less deformity, athletic shoes with sufficient room for the toes and forefoot and cushioned socks are appropriate.
Foot ulcers. Detection and early treatment of foot ulcers is of paramount importance. Foot ulcers are among the most common reason for hospitalization in people with diabetes and are the leading cause of lower extremity amputations. However, evidence suggests that up to 85% of amputations are avoidable with patient education, medical monitoring, and early intervention.90 Careful evaluation of vascular status and infection are required upon discovery of an ulcer. Initiate early treatment with aggressive wound care, antibiotics, revascularization, orthotic prescriptions, and casting to offload the ulcer when appropriate. Studies have shown patients with diabetic foot ulcers have the best outcomes if managed by a multidisciplinary team that specializes in diabetic foot care. Hyperbaric oxygen therapy may be recommended in managing diabetic foot ulcers, although trials have shown mixed results.
Treatment of painful diabetic peripheral neuropathy (PDN)
Recommendations
Optimize glycemic control to reduce progression of PDN.
Check for vitamin B12 deficiency in patients with chronic metformin use.
Treat painful diabetic peripheral neuropathy with pregabalin, duloxetine or gabapentin which are first line therapies and FDA approved for treatment of PDN.
Less preferred medications with some evidence of benefit but significant potential for side effects:
- Tricyclic antidepressants but use with caution in elderly patients due to anticholinergic side effects. Nortriptyline is preferred.
- Carbamazepine, valproate, topical capsaicin cream or lidocaine patch. Use is limited due to side effects.
- Use of opioids is last resort, but discouraged as evidence is inconclusive, with potential harms likely outweighing benefits.
- Non-pharmacological approach such as acupuncture, TENS.
Optimizing glycemic control is of paramount importance in slowing the progression of established diabetic neuropathy. Consider checking and treating vitamin B12 level in patients who use chronic metformin as vitamin B12 deficiency can contribute to worsening of neuropathy.
NSAIDS should not be used for chronic neuropathic pain as they are ineffective and increase cardiovascular risk, GI, and renal side effects. Long term NSAID treatment increases the risk of GI bleeding and renal insufficiency. NSAID use in patients with heart disease or its risk factors increases overall risk of heart attack or stroke.91
First line therapies for the treatment of PDN supported by the literature include pregabalin, duloxetine, and gabapentin. Pregabalin and duloxetine have FDA approval for treatment of neuropathic pain in diabetes. Tricyclic antidepressants (TCAs) are also options. Comparative effectiveness studies and trials that include quality of life outcomes are rare – so treatment decisions must consider patient’s comorbidities, presentation, symptom improvement, medication adherence, and side effects.
- Pregabalin (up to 300-450 mg/day as divided doses) is FDA-approved and is less sedating. Most extensively studied and have favorable effects with at least 30-50% improvement in pain. Starting at 25-75 mg once daily or in divided doses then titrating up to lowest effective tolerable dose will minimize side effects especially in elderly patients.
- Duloxetine (60-120 mg/day) and venlafaxine (75-450 mg/day), serotonin and norepinephrine reuptake inhibitors (SNRIs) are useful in treating patients with co-morbid depression. Duloxetine is FDA-approved. It has been shown to improve neuropathy-related quality of life. Selective Serotonin Reuptake Inhibitors (SSRIs) and trazodone are not as effective in treating painful PDN.
- Gabapentin up to 900-3600 mg/day as divided doses, or more may be required. Use lowest effective dose. Sedation is a side effect that limits its use. May be a less expensive option.
- TCAs may be used to treat painful neuropathy. Use with caution in the elderly and start with low doses and titrate to maximize pain relief while minimizing side effects. Most common side effects include: dry mouth, sedation, orthostatic hypotension and constipation. Nortriptyline is the preferred tricyclic as it has fewer anticholinergic properties. Recommend initiating with dinner, a dose of 10-25 mg and titrate up as tolerated, to maximum of 150 mg/day.
Other agents. Carbamazepine (200 – 600 mg/day) and valproate (500 mg/day), topical capsaicin cream, and lidocaine patch have been shown to decrease PDN. Their use is limited by their side effect profiles.
Opioids. Tapentadol Extended Release is FDA approved for the treatment of neuropathic pain associated with diabetes based on data from two multicenter clinical trials but the design included patients who responded to tapentadol and therefore is not generalizable. As a last resort, opioids may be considered, though general use is discouraged given high risk for addiction and safety concerns compared to the relatively modest pain reduction. Tramadol is a weak opioid and dose of 37.5 mg with 325 mg acetaminophen showed an improvement in PDN compared to placebo. Refer to the UMHS Clinical Care Guideline “Managing Chronic Non-Terminal Pain in Adults Including Prescribing Controlled Substances“.
Acupuncture and TENS. Several studies have shown the efficacy of using traditional acupuncture for the treatment of painful diabetic neuropathy. Transcutaneous Electrical Nerve Stimulation (TENS) has also been evaluated and has been shown to reduce lower extremity pain associated with PDN. Hyperbaric oxygen therapy as an adjunct therapy to standard treatment has been shown to reduce cell death, pain symptoms and rates of major amputations in patients with diabetic foot ulcers and peripheral arterial occlusive disease.
Immunizations
Patients with diabetes should get the usual vaccinations recommended for the general population. Particular attention should be paid to influenza vaccination (annually) and Hepatitis B due to the increased risks for these diseases in patients with diabetes. In addition, patients with diabetes, regardless of their age, should be given Pneumovax 23 for pneumococcal pneumonia. If vaccination occurred prior to the patient turning age 65, a second dose is required at least 5 years after receiving the first dose. Patients over age 65, who were not previously vaccinated should receive one dose of Pneumovax 23.
Complementary and Alternative Therapies
Recommendations
Yoga, tai chi, and acupuncture should be encouraged.
Individuals with diabetes are using complementary and alternative (CAM) therapies in ever-increasing numbers. Often, the health care provider is unaware of such use, and such interventions may interact with conventional therapy, for example the addition of a glucose-lowering herbal supplement to a sulfonylurea leading to hypoglycemia. The importance of asking individuals which supplements or complementary therapies they use cannot be overemphasized.
Pharmacologic CAM therapies have been studied. A number of traditionally used supplements have shown promise for the treatment of diabetes. However, limitations for these studies are their short duration, small sample size, poor methodology, and lack of reporting of clinically important outcomes.
Supplementation with multivitamins is generally considered safe; however, megavitamin therapy should be discouraged. Relaxation therapy, yoga, tai chi, acupuncture and spiritual healing are helpful to individuals and can be encouraged. Interventions that are potentially harmful or have no real evidence of efficacy clearly should be discouraged. Patients should be commended, however, on their self-determination and encouraged to direct their efforts in areas that have proven benefits.
Special Populations
Diabetes in Women
Recommendations
Preconception:
- Screen women that are planning to get pregnant and are at high risk for type 2 diabetes prior to conception.
- Women with type 2 diabetes should be aware of the need for preconception counseling prior to planning pregnancy.
- Discuss and review contraceptive usage and pregnancy plans at each visit for women with type 2 diabetes.
- - Explain the rationale of continuing contraception until goal A1c is achieved.
Pregnant women without diagnosis of type 2 diabetes:
- Refer women with a fasting glucose ≥95 mg/dl to the GDM program run by the Adult Diabetes Education Program and MEND.
Pregnancy with type 2 diabetes:
- Insulin is the preferred medication for pregnancies complicated by diabetes.
- Check A1c urgently in women with T2DM and newly diagnosed pregnancy.
- Those with an A1c 5.7-6.4% and a fasting glucose <95 should be screened early for gestational diabetes.
- Refer those with an A1c ≥6.5% or a fasting glucose ≥126 mg/dL to high risk obstetrics urgently and to a gestational diabetes education program.
- Review glucose records and adjust insulin at least every 1-2 weeks during pregnancy, due to the physiologic changes in insulin requirements during pregnancy
Glycemic Targets in pregnancy:
- Fasting < 95 mg/dL (5.3 mmol/L)
- Two-hour postprandial <120 mg/dL (6.7 mmol/L)92
- A1c <6.0%, if it can be achieved safely
Blood Pressure Targets in pregnancy:
On exam, any pregnant patient without a history of hypertension and a BP >140/90, or with a known history of hypertension and a BP >150/100 is at risk. This necessitates an interventional plan and an urgent discussion with the obstetrical provider.
Post-partum women:
- Insulin requirements drop dramatically (~1/2 of pre-pregnancy) at delivery for women with pre-existing diabetes.93
- Screen for type 2 diabetes in patients where gestational diabetes resolved after delivery, annually by a fasting glucose, A1c, or OGTT (every 3 years).
An increasing number of premenopausal women have T2DM (~1%) which complicates decisions about contraception, preconception counseling, and management of pregnancy. Many are unaware of their diagnosis and its implications on pregnancy. In addition, another ~10% of women will manifest gestational diabetes (GDM) during pregnancy, indicating an inability of the pancreas to expand insulin production, which significantly increases the patient’s risk of developing T2DM within 10 years.
Contraception
Approximately half of all pregnancies are unplanned, therefore, it is imperative that women with diabetes plan for their pregnancies and achieve an A1c < 6.5% prior to conception. The A1c at the time of conception is predictive of congenital malformations and other complications. Effective contraception is the first step in planning a successful pregnancy in women with diabetes. All women of child-bearing age with diabetes should have contraceptive usage and pregnancy plans reviewed at each visit, including at all their primary care, OB/GYN, and endocrinology appointments. No specific contraceptive choice is recommended, or contraindicated in women with diabetes, however reliability is important. Long-acting Reversible Contraception (LARC), such as an intrauterine device or subdermal implant, are the most effective reversible contraceptive methods available, should be considered for all patients
Preconception Counseling and Care
Women with diabetes should be educated on the risks of diabetes and pregnancy. Those who are considering pregnancy should receive preconception counseling and care (see checklist below).
Check list for Preconception Care/Counseling
Education and counselling
- Relationship between A1c at the time of conception and through pregnancy and pregnancy outcomes, including congenital malformations, still birth and autism.
- Relationship between A1c at the time of conception and throughout pregnancy with maternal complications, including vision loss, preeclampsia and kidney failure.
- Comprehensive diabetes education, if not previously performed, and targeted updates on nutrition and pregnancy.
- Counseling on obesity and pregnancy, if indicated.
Specific care recommendations for:
- Referral for a Maternal Fetal Medicine consult (UMHS).
- Referral to the MEND Endocrine Disorders in Pregnancy program for preconception care to optimize A1c is recommended.
- Initiation of Prenatal vitamins.
- Screening for diabetes complications and comorbidities, including thyroid disease.
- Optimization of medications for diabetes and diabetes comorbidities for pregnancy.
- – ACE inhibitors, ARBs and spironolactone should be discontinued and if antihypertensives are needed, labetalol or nifedipine are preferred. Atenolol should be avoided.
- – Statins and other lipid lowering medications should be discontinued.
- Referral for a comprehensive eye exam.
- Initiation of baby aspirin (162 mg daily) to reduce the risk of preeclampsia.
The importance of preconception counselling and care, especially the optimization of the A1c cannot be overstated. In an analysis of the TRUVEN database, pregnancies in women with type 2 diabetes were complicated by miscarriage (25.2% of pregnancies), stillbirth (0.8%), major congenital malformation (10.9%), and congenital heart defect (6.9%).98 Often these complication rates were higher than in pregnancies complicated by type 1 diabetes, in part due to a higher level of exposure to diabetes education in women with type 1 diabetes. Preconception counselling and optimal A1c in the first trimester have been shown to reduce the risk of congenital malformations and other pregnancy complications dramatically in multiple studies.
Screening and Diagnosis for GDM
Recent studies have shown an increased prevalence of type 1, type 2, and GDM during pregnancy in both the US and worldwide. Given the risks associated with diabetes and pregnancy, it is reasonable to screen for diabetes in women at high risk prior to conception. Most congenital malformations from hyperglycemia occur before women are even aware they are pregnant.
Michigan Medicine now recommends screening for undiagnosed diabetes and prediabetes at the first prenatal visit, using a fasting glucose and A1c. A fasting glucose ≥ 95 mg/dL is considered diagnostic of GDM. Patients with A1c in the prediabetes range (5.7-6.4) are at high risk for GDM and should be screened promptly for gestational diabetes with a 50 gm oral glucose challenge test, although it may be reasonable in some patients to establish a diagnosis of GDM based on this criterion alone.
Treatment of DM in pregnancy
Treatment of GDM and pre-existing diabetes has been shown to reduce the risk of macrosomia, preeclampsia, and other known complications of diabetes in pregnancy. Optimization of medical nutrition therapy, exercise, sleep, and emotional wellbeing are essential components in the management of all types of diabetes.
Glycemic targets in pregnancy are:
- Fasting < 95 mg/dL (5.3 mmol/L)
- Two-hour postprandial <120 mg/dL (6.7 mmol/L)92
Many women with GDM can achieve glycemic targets in pregnancy with the comprehensive lifestyle coaching. Michigan Medicine recommends all women diagnosed with GDM be referred to the GDM program.
Insulin is the preferred medication for treating diabetes during pregnancy, given it does not typically cross the placenta. Most women with preexisting diabetes require basal bolus therapy to achieve targets, however, some with GDM can achieve goals with once daily bedtime insulin, or mixed insulins dosed before both breakfast and dinner. Most oral medications for diabetes are not recommended in pregnancy. However, do not stop oral medication for diabetes in the first trimester, until insulin has been initiated. Urgently refer those patients to their endocrinologist.
All forms of diabetes are considered risk factors for the development of preeclampsia. A high degree of suspicion is imperative. Page the obstetric care provider (or if unavailable, the MFM on call) for any pregnant patient with no history of hypertension and a BP >140/90; or those with a known history of hypertension and a BP > 150/100.
Monitoring
Self-monitored blood glucose is the mainstay of glycemic monitoring in pregnancy. Patients should check their glucose level 4-7 times a day. Both fasting and postprandial sugars should be followed on all patients. Some patients may require glucose monitoring before lunch, dinner, and at bedtime. Postprandial monitoring is recommended because it is associated with better glycemic control and lower risk of macrosomia and preeclampsia.99–101
A1c may not fully capture hyperglycemia and hypoglycemia, therefore should not be used as a primary measure of glycemic control in pregnancy. A1c values <6% during pregnancy are associated with the lowest risk of complications. Frequent hypoglycemia may increase the risk of small for gestational age infants.
CGM technology has been shown to help achieve glycemic targets in type 1 diabetes and pregnancy in CONCEPPT.102 Data in type 2 diabetes and GDM is lacking. CONCEPPT was a RCT of CGM which demonstrated a reduction in LGA and neonatal hypoglycemia, as well as improvement in A1c without an increase in hypoglycemia with CGM use. It is important to note that in both arms of CONCEPPT, patients used SBGM pre and post meals and used that information to achieve pre- and postprandial targets. CGM metrics were not used as outcomes, or substitutes for traditional postprandial targets. Therefore, CGM metrics should not be used as a substitute for fasting and postprandial targets.
Glucose records should be reviewed and insulin adjustments made at least every 1-2 weeks during pregnancy given the physiologic changes in insulin requirements during pregnancy.
Postpartum care
Post-partum is a time of great change in a woman’s life and offering psychosocial support during this time is important. Discussing and implementing a new contraceptive plan is a critical element of postpartum care. Also emphasizing preconception counselling for future pregnancies is essential.
For women with pre-existing diabetes, it is important to realize that insulin requirements drop dramatically at delivery, often to roughly ½ the pre-pregnancy requirements.93 The first few weeks post-partum are a time to be especially cautious of severe hypoglycemia. Supporting breast feeding is sometimes challenging in women with diabetes as insulin often has to be adjusted to compensate for increased overnight hypoglycemia.
For patients with GDM, there is a 50% risk of progression to type 2 diabetes within 5-10 years after delivery, as well as increased risk for cardiovascular disease. For women with GDM, life-long screening for type 2 diabetes is recommended, starting with an OGTT at 4-12 weeks post-partum and continuing with either OGTT, fasting glucose, or A1c at least every 3 years. Referral to a National Diabetes Prevention Program is recommended with a goal of achieving weight loss of at least 7% of the pre-pregnancy weight and achieving at least150 minutes of exercise weekly.
Older Individuals with Diabetes
Recommendations
Decisions regarding A1c goal, blood pressure goal, use of screening for complications, and use of lipid lowering drugs should be individualized.
Use glucose lowering drugs that are not commonly associated with hypoglycemia.
De-intensify glucose-lowering drug regimens within the individualized A1c goal, when possible.
Avoid symptomatic hyperglycemia, especially when a more relaxed A1c goal set.
Screen for cognitive impairment beginning at age 65.
Patients receiving palliative care do not require strict blood pressure and glucose control; and lipid lowering therapy may be withdrawn.
Evidence is lacking for high quality studies to help guide management of diabetes in older adults, yet this is a population that presents unique challenges that require special attention. This population highlights the need for individualized care due to the heterogeneity in comorbidities, life expectancy, vitality, and patient preferences. Diabetes substantially increases the risk of developing dementia, fragility fractures, depression, falls, and urinary incontinence. Carefully screen for these conditions in older populations.
Older patients with diabetes are at increased risk for hypoglycemia and its complications. Hypoglycemia increases the risk for cognitive impairment and other adverse outcomes. Prioritize the avoidance of hypoglycemia and prescribe glucose lowering drugs which are not associated with inducing hypoglycemia.
Individualize A1c goals for older patients. Those who are otherwise healthy, with few coexisting chronic illnesses, have intact cognitive function, and functional status should have lower glycemic goals (eg, A1c <7.5%). Those with multiple coexisting chronic illnesses, cognitive impairment, or functional dependence should have less-stringent glycemic goals (eg, A1c <8.0 – 8.5%). Table 4 discusses A1c targets. De-intensification of glucose lowering regimens is recommended provided it can be accomplished within the individualized A1c goal. Symptomatic hyperglycemia should be avoided especially when a more relaxed A1c goal has been selected.
Controlling other cardiovascular risk factors beyond hyperglycemia may help to reduce cardiovascular events in older individuals. A 2008 RCT demonstrated that using antihypertensives to achieve a goal BP of less than 150/90 reduces risk of MI and stroke. There are no trials to date that have specifically examined older individuals’ use of lipid-lowering therapy, but such therapy may be warranted for primary prevention of atherosclerotic disease events when the individual’s life expectancy is comparable to the study duration, which is about five years.
When palliative care has been implemented, strict glucose and blood pressure control are usually not warranted. Discontinuation of lipid-lowering medications may be warranted in this situation as well.
Coordination with Other Care Providers
How to adjust diabetes medication for procedures and surgeries is a common question for patients with diabetes and their health care providers. Table 13 summarizes these recommendations.
Table 14 list reasons to consider referral to Endocrinology.
Table 14
When to Consider Endocrine Consultation or Referral.
Pre-procedure and Pre-Operative Diabetes Medication Adjustment Guidelines
Pre-procedure and Pre-operative Diabetes Medication Adjustment Guidelines are in the table below
Guideline Development Methodology
Funding
The development of this guideline was funded by UMHS.
Guideline Development Team and Disclosures
The multidisciplinary guideline development team consisted of:
- Caroline R Richardson, MD, Family Medicine; Jeffrey R Borgeson, MD, General Internal Medicine; Jennifer A Wyckoff, MD, Metabolism, Endocrinology & Diabetes; Anne S Yoo, PharmD, Pharmacy Innovations and Partnerships. Consultants: James E Aikens, PhD, Family Medicine; Dina H Griauzde, MD, General Internal Medicine; Monica A Tincopa, MD, MSc, Gastroenterology.
- Guideline development methodologists: R. Van Harrison, PhD, Learning Health Sciences, April Proudlock, RN Clinical Quality.
- Literature search services were provided by informationists at the Taubman Health Sciences Library, University of Michigan Medical School.
UMHS endorses the Standards of the Accreditation Council for Continuing Medical Education that the individuals who present educational activities disclose significant relationships with commercial companies whose products or services are discussed. Contributions of team members with relevant financial relationships are reviewed by team members without relevant financial relationships to assure the information is presented without bias.
Individuals with no relevant personal financial relationships:
James E Aikens, PhD, Jeffrey R Borgeson, MD, R Van Harrison, PhD, Karl T Rew, MD, Jennifer A Wyckoff, MD, Anne S Yoo, PharmD
Individuals with relevant personal financial relationships:
Caroline R Richardson, MD:
- Grant funding from industry – completed in last year - Apple, Dexcom, Twine
- Grant funding from industry – ongoing –Blue Cross Blue Shield
- Grant funding from industry being negotiated, potential – Renalytics
Dina Griauzde, MD
- Consultant – National Kidney Foundation of Michigan
Systematic Review of Literature
The team began the search of literature by accepting the results of a systematic literature review performed in 1995 to develop the guideline and again in 2003 and 2010 for major updates.:
VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care. Washington, DC: U.S. Departments of Veterans Affairs and of Defense, 2017. Search results performed through 3/25/16. (Topic specific search terms, pages 141-147.)
To update those results, we performed a systematic search of literature on Medline and in the Cochrane Database of Systematic Reviews for the time period 3/1/16 – 3/1/19.
The major search term was diabetes mellitus. The searches were for guidelines, controlled trials (including meta-analyses), and cohort studies, for literature on humans in the English language. Within these parameters individual searches were performed for the following topics:
- Prevention: Include drug-induced diabetes
- Screening: Diabetes, prediabetes
- Diagnosis: History (risk factors, symptoms), physical exam
- Diabetes self-management: Exercise, meal planning and nutrition, medication adherence, smoking cessation, insurance, cost
- Glycemic Control: Treatment, glucose monitoring, glycemic management, pharmacologic glucose management, insulin, medications that increase blood sugar
- Co-morbid medical conditions: Hypertension, treatment, non-alcoholic fatty liver disease (NAFLD), obesity, obstructive sleep apnea
- Other comorbid conditions not included in F above
- Psychological comorbidities and complications: Screening and treatment
- Macrovascular complications: Screening and treatment, stroke risk, CAD risk, autonomic neuropathy and cardiovascular disease
- Microvascular complications: Screening and treatment, retinopathy, nephropathy, neuropathy, diabetic foot care, first line therapies, other agents
- Immunizations: Influenza, pneumococcal, hepatitis b
- Complementary and alternative therapies
- Special populations: Adolescents, preconception, pregnant women, gestational diabetes, older individuals
- Other not in A-M above
The search was conducted in components each keyed to a specific causal link in a formal problem structure. The search was supplemented with very recent controlled trials known to expert members of the panel. Negative trials were specifically sought. The search was single cycle. Conclusions were based on prospective randomized controlled trials if available, to the exclusion of other data. If randomized controlled trials were not available, observational studies were admitted to consideration. If no such data were available for a given link in the problem formulation, expert opinion was used to estimate effect size. The “strength of recommendation” for key aspects of care was determined by expert opinion.
Literature review and assessment: Members of the guideline team reviewed the publications identified to be relevant to specific topics in order to select those with best evidence. Criteria to identify overall best evidence included relevance of the study setting and population, study design, sample size, measurement methods (variables, measures, data collection), intervention methods (appropriateness, execution), appropriateness of analyses, and clarity of description.
In considering level of evidence based on study design, the classification was:
- A = systematic reviews of randomized controlled trials with or without meta-analysis
- B = randomized controlled trials
- C = systematic reviews of non-randomized controlled trials or observational studies, non-randomized controlled trials, group observation studies (cohort, cross-sectional, case-control)
- D = individual observation studies (case study or case series)
- E = expert opinion regarding benefits and harm
Recommendations. The guideline team reviewed the evidence and determined the importance of performing or not performing key aspects of care (listed on the first page of this guideline). In the absence of empirical evidence, the guideline team based recommendations on their expert opinion.
The strength of recommendations regarding care were categorized as:
- I = Generally should be performed
- II = May be reasonable to perform
- III = Generally should not be performed
Review and Endorsement
Drafts of this guideline were reviewed in clinical conferences and by distribution for comment within departments and divisions of the University of Michigan Medical School to which the content is most relevant: Family Medicine; General Medicine; Geriatric Medicine; and Metabolism, Endocrinology, and Diabetes. The draft was revised based on comments from these groups.
The final version of this guideline was endorsed by the Clinical Practice Committee of the University of Michigan Medical Group and by the Executive Committee for Clinical Affairs of the University of Michigan Hospitals and Health Centers.
Acknowledgments
The following individuals are acknowledged for their contributions to previous versions of this guideline.
2019: Connie J Standiford, MD, General Internal Medicine, Sandeep Vijan, MD, General Internal Medicine, Hae Mi Choe, PharmD, College of Pharmacy, R Van Harrison, PhD, Medical Education, Caroline R Richardson, MD, Family Medicine, Jennifer A Wyckoff, MD, Metabolism, Endocrinology & Diabetes. Consultants: Martha M Funnell, MS, RN, CDE, Diabetes Research and Training Center, William H Herman, MD, Metabolism, Endocrine & Diabetes.
2012: Connie J Standiford, MD, General Internal Medicine, Sandeep Vijan, MD, General Internal Medicine, Hae Mi Choe, PharmD, College of Pharmacy, R Van Harrison, PhD, Medical Education, Caroline R Richardson, MD, Family Medicine, Jennifer A Wyckoff, MD, Metabolism, Endocrinology & Diabetes. Consultants: Martha M Funnell, MS, RN, CDE, Diabetes Research and Training Center, William H Herman, MD, Metabolism, Endocrine & Diabetes.
2004: Deryth L Stevens, MD, Family Medicine, Sandeep Vijan, MD, General Internal Medicine, Martha M Funnell, MS, RN, Diabetes Research and Training Center, R Van Harrison, PhD, Medical Education, William H Herman, MD, Endocrinology and Metabolism, Robert W Lash, MD, Endocrinology and Metabolism.
1996: Deryth Stevens, MD, Family Medicine, Sandeep Vijan, MD, General Internal Medicine, Martha Funnell, MS, RN, Diabetes Research and Training Center, Douglas Greene, MD, Endocrinology and Metabolism, R. Van Harrison, PhD, Postgraduate Medicine, William Herman, MD, Endocrinology and Metabolism, Roland Hiss, MD, Postgraduate Medicine, Catherine Martin, MS, RN, Endocrinology and Metabolism, Evelyn Piehl, MS, RN, Obstetrics and Gynecology, B.J. Ratliff, RN, Primary Care Nursing, Connie Standiford, MD, General Internal Medicine.
References
- 1.
- Holt RIG. Association Between Antipsychotic Medication Use and Diabetes. Curr Diab Rep. 2019;19(10). doi:10.1007/s11892-019-1220-8 [PMC free article: PMC6718373] [PubMed: 31478094] [CrossRef]
- 2.
- Georgianos PI, Agarwal R. Ambulatory blood pressure reduction with SGLT-2 inhibitors: Dose-Response Meta-analysis and Comparative Evaluation With Low-Dose Hydrochlorothiazide. Diabetes Care. 2019;42(4):693–700. doi:10.2337/dc18-2207 [PMC free article: PMC6429633] [PubMed: 30894383] [CrossRef]
- 3.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Vol 139.; 2019. doi:10.1161/CIR.0000000000000624 [PubMed: 30565953] [CrossRef]
- 4.
- Professional Practice Committee. Standards of Medical Care in Diabetes. Diabetes Care. 2019;Jan(42, Supplement 1):1–204. [PubMed: 30559225]
- 5.
- Ballestri S, Nascimbeni F, Lugari S, Lonardo AFG. A critical appraisal of the use of ultrasound in hepatic steatosis. Expert Rev Gastroenterol Hepatol. 2019;13(7):677–681. [PubMed: 31104523]
- 6.
- Parry S, Hodson L. Managing NAFLD in Type 2 Diabetes: The Effect of Lifestyle Interventions, a Narrative Review. Adv Ther. 2020;37(4):1381–1406. [PMC free article: PMC7140753] [PubMed: 32146704]
- 7.
- Moosavian, S. P., Arab, A., & Paknahad Z. The effect of a Mediterranean diet on metabolic parameters in patients with non-alcoholic fatty liver disease: A systematic review of randomized controlled trials. Clin Nutr ESPEN. 2019;35:40–46. [PubMed: 31987120]
- 8.
- D’abbondanza M, Ministrini S, Pucci G, et al. Very low-carbohydrate ketogenic diet for the treatment of severe obesity and associated non-alcoholic fatty liver disease: The role of sex differences. Nutrients. 2020;12(9):1–14. doi:10.3390/nu12092748 [PMC free article: PMC7551320] [PubMed: 32916989] [CrossRef]
- 9.
- Lv X, Dong Y, Hu L, Lu F, Zhou C, Qin S. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) for the management of nonalcoholic fatty liver disease (NAFLD): A systematic review. Endocrinol Diabetes Metab. 2020;3(3):1–16. doi:10.1002/edm2.163 [PMC free article: PMC7375121] [PubMed: 32704576] [CrossRef]
- 10.
- Xing B, Zhao Y, Dong B, Zhou Y, Lv W, Zhao W. Effects of sodium–glucose cotransporter 2 inhibitors on non-alcoholic fatty liver disease in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. J Diabetes Investig. 2020:1–10. doi:10.1111/jdi.13237 [PMC free article: PMC7477503] [PubMed: 32083798] [CrossRef]
- 11.
- Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342–362. doi:10.1210/jc.2014-3415 [PubMed: 25590212] [CrossRef]
- 12.
- Lazarus E. Practical Medication Management for Obesity Treatment. http://www
.omacademy .org/store/seminar/seminar .php?seminar=72626. - 13.
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63(25 PART B):2985–3023. doi:10.1016/j.jacc.2013.11.004 [PubMed: 24239920] [CrossRef]
- 14.
- Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes. 2012;36(6):843–854. doi:10.1038/ijo.2011.158 [PMC free article: PMC3374073] [PubMed: 21844879] [CrossRef]
- 15.
- Report NDS. National Diabetes Statistics Report, 2020. Natl Diabetes Stat Rep. 2020:2.
- 16.
- Li G, Zhang P, Wang J et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371(9626):1783–1789. [PubMed: 18502303]
- 17.
- Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, Hämäläinen H, Härkönen P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Mannelin M, Paturi M, Sundvall J, Valle TT, Uusitupa M TJFDPSG. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;11(368):1673–1679. [PubMed: 17098085]
- 18.
- Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD VVIDPP (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49(2):289–297. [PubMed: 16391903]
- 19.
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA NDDPPRG. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;7(364):393–403. [PMC free article: PMC1370926] [PubMed: 11832527]
- 20.
- Uusitupa M, Khan T, Viguiliouk E, Kahleova H. Prevention of Type 2 Diabetes by Lifestyle Changes: Nutrients. 2019;11(2611):1–22. [PMC free article: PMC6893436] [PubMed: 31683759]
- 21.
- Association AD. 3. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S32–S36. [PubMed: 31862746]
- 22.
- Steinsbekk A, Rygg LØ, Lisulo M, By Rise M, Fretheim A. Group based diabetes self-management education compared to routine treatment, waiting list control or no intervention for people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2015;(6). doi:10.1002/14651858.CD003417.pub3 [CrossRef]
- 23.
- Bian RR, Piatt GA, Sen A, et al. The Effect of Technology-Mediated Diabetes Prevention Interventions on Weight: A Meta-Analysis. J Med Internet Res. 2017;19(3):e76. doi:https://dx
.doi.org/10.2196/jmir.4709 [PMC free article: PMC5387112] [PubMed: 28347972] - 24.
- Sallar A, Dagogo-Jack S. Regression from prediabetes to normal glucose regulation: State of the science. Exp Biol Med. 2020;245(10):889–896. doi:10.1177/1535370220915644 [PMC free article: PMC7268926] [PubMed: 32212859] [CrossRef]
- 25.
- Anderson RJ, Freedland KE, Clouse RELP. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069–1078. doi:10.1111/j.1464-5491.2006.01943.x [PubMed: 11375373] [CrossRef]
- 26.
- De Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: A meta-analysis. Psychosom Med. 2001;63(4):619–630. doi:10.1097/00006842-200107000-00015 [PubMed: 11485116] [CrossRef]
- 27.
- Young-Hyman D, De Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: A position statement of the American diabetes association. Diabetes Care. 2016;39(12):2126–2140. doi:10.2337/dc16-2053 [PMC free article: PMC5127231] [PubMed: 27879358] [CrossRef]
- 28.
- Semenkovich K, Brown ME, Svrakic DM, Lustman PJ. Depression in type 2 diabetes mellitus: Prevalence, impact, and treatment. Drugs. 2015;75(6):577–587. doi:10.1007/s40265-015-0347-4 [PubMed: 25851098] [CrossRef]
- 29.
- Fisher L, Glasgow RE, Mullan JT, Skaff MM, Polonsky WH. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6(3):246–252. doi:10.1370/afm.842 [PMC free article: PMC2384991] [PubMed: 18474888] [CrossRef]
- 30.
- Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018;6(2):122–129. doi:10.1016/S2213-8587(17)30362-5 [PMC free article: PMC5805861] [PubMed: 29199115] [CrossRef]
- 31.
- Pozzilli P, Pieralice S. Latent autoimmune diabetes in adults: Current status and new horizons. Endocrinol Metab. 2018;33(2):147–159. doi:10.3803/EnM.2018.33.2.147 [PMC free article: PMC6021307] [PubMed: 29947172] [CrossRef]
- 32.
- Thomas NJ, Lynam AL, Hill AV, et al. Type 1 diabetes defined by severe insulin deficiency occurs after 30 years of age and is commonly treated as type 2 diabetes. 2019:1167–1172. [PMC free article: PMC6559997] [PubMed: 30969375]
- 33.
- Andersen DK, Korc M, Petersen GM, et al. Diabetes, pancreatogenic diabetes, and pancreatic cancer. Diabetes. 2017;66(5):1103–1110. doi:10.2337/db16-1477 [PMC free article: PMC5399609] [PubMed: 28507210] [CrossRef]
- 34.
- Serfaty L, Capeau J. Hepatitis C, insulin resistance and diabetes: Clinical and pathogenic data. Liver Int. 2009;29(SUPPL. 2):13–25. doi:10.1111/j.1478-3231.2008.01952.x [PubMed: 19187069] [CrossRef]
- 35.
- Catargi B, Rigalleau V, Poussin A, et al. Occult Cushing’s Syndrome in Type-2 Diabetes. J Clin Endocrinol Metab. 2003;88(12):5808–5813. doi:10.1210/jc.2003-030254 [PubMed: 14671173] [CrossRef]
- 36.
- Abraham A, Barnett C, Katzberg HD, Lovblom LE, Perkins BA, Bril V. High frequency of MGUS in DSP. Muscle and Nerve. 2018;57(6):1018–1021. doi:10.1002/mus.26054 [PubMed: 29314079] [CrossRef]
- 37.
- Cai J, Wang Y, Baser O, Xie L, Chow W. Comparative persistence and adherence with newer anti-hyperglycemic agents to treat patients with type 2 diabetes in the United States. J Med Econ. 2016;19(12):1175–1186. [PubMed: 27356271]
- 38.
- Iglay K, Cartier SE, Rosen VM, et al. Meta-analysis of studies examining medication adherence, persistence, and discontinuation of oral antihyperglycemic agents in type 2 diabetes. Curr Med Res Opin. 2015;31(7):1283–1296. doi:10.1185/03007995.2015.1053048 [PubMed: 26023805] [CrossRef]
- 39.
- Couto JE, Panchal JM, Lal LS, et al. Geographic variation in medication adherence in commercial and medicare part D populations. J Manag Care Pharm. 2014;20(8):834–842. doi:10.18553/jmcp.2014.20.8.834 [PMC free article: PMC10438096] [PubMed: 25062077] [CrossRef]
- 40.
- Bussell JK, Cha ES, Grant YE, Schwartz DD, Young LA. Ways health care providers can promote better medication adherence. Clin Diabetes. 2017;35(3):171–177. doi:10.2337/cd016-0029 [PMC free article: PMC5510928] [PubMed: 28761220] [CrossRef]
- 41.
- Krass I, Schieback P, Dhippayom T. Adherence to diabetes medication: A systematic review. Diabet Med. 2015;32(6):725–737. doi:10.1111/dme.12651 [PubMed: 25440507] [CrossRef]
- 42.
- Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470–482. doi:10.1007/s13142-015-0315-2 [PMC free article: PMC4656225] [PubMed: 26622919] [CrossRef]
- 43.
- McHorney CA. The Adherence Estimator: A brief, proximal screener for patient propensity to adhere toprescription medications for chronic disease. Curr Med Res Opin. 2009;25(1):215–238. doi:10.1185/03007990802619425 [PubMed: 19210154] [CrossRef]
- 44.
- Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: A systematic review. Ann Intern Med. 2012;157(11):785–795. doi:10.7326/0003-4819-157-11-201212040-00538 [PubMed: 22964778] [CrossRef]
- 45.
- Barer Y, Cohen O, Cukierman-Yaffe T. Effect of glycaemic control on cardiovascular disease in individuals with type 2 diabetes with pre-existing cardiovascular disease: A systematic review and meta-analysis. Diabetes Obes Metab. 2019;21(3):732–735. doi:https://dx
.doi.org/10.1111/dom.13581 [PubMed: 30426626] - 46.
- Maiorino MI, Signoriello S, Maio A et al. Effects of Continuous Glucose Monitoring on Metrics of Glycemic Control in Diabetes: A Systematic Review With Meta-analysis of Randomized Controlled Trials. Diabetes Care. 2020;43(5):1146–1156. [PubMed: 32312858]
- 47.
- Wexler DJ, Krause-Steinrauf H, Crandall JP, et al. Baseline characteristics of randomized participants in the glycemia reduction approaches in diabetes: A comparative effectiveness study (GRADE). Diabetes Care. 2019;42(11):2098–2107. doi:10.2337/dc19-0901 [PMC free article: PMC6804613] [PubMed: 31391203] [CrossRef]
- 48.
- Matthews DR, Li Q, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Desai M, Hiatt WR, Nehler M, Fabbrini E, Kavalam M, Lee MNB. Effects of canagliflozin on amputation risk in type 2 diabetes: the CANVAS Program. Diabetologia. 2019;62(6):926–938. [PMC free article: PMC6509073] [PubMed: 30868176]
- 49.
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. Arias P Alvarisqueta A, Maffei L, Fretes JO, De Lapertosa SG, Visco V, Sposetti G, Farias J, Farias EF, Cantero MC, Feldman R, Ridruejo MC, Calella P, Zaidman C, Stranks S, Mah PM, Nankervis A, Topliss D, Soldatos G, Simpson R, Gerstman M, Colquhoun D, MUMR, ed. N Engl J Med. 2017;377(7):644–657. doi:https://dx
.doi.org/10.1056/NEJMoa1611925 [PubMed: 28605608] - 50.
- Lo KB, Gul F, Ram P, Kluger AY, Tecson KM, McCullough PARJ. The Effects of SGLT2 Inhibitors on Cardiovascular and Renal Outcomes in Diabetic Patients: A Systematic Review and Meta-Analysis. Cardiorenal Med. 2020;10(1):1–10. [PubMed: 31743918]
- 51.
- Rosenstock JFE. Euglycemic Diabetic Ketoacidosis: A Predictable, Detectable, and Preventable Safety Concern With SGLT2 Inhibitors. Diabetes Care. 2015;38(9):1638–1642. [PubMed: 26294774]
- 52.
- Rosenstock J, Perkovic V, Johansen OE, et al. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA. 2019;321(1):69–79. doi:https://dx
.doi.org/10 .1001/jama.2018.18269 [PMC free article: PMC6583576] [PubMed: 30418475] - 53.
- Khwaja NUDAG. Efficacy and cardiovascular safety of Alpha glucosidase inhibitors. Curr Drug Saf. 2020;16. [PubMed: 33334296]
- 54.
- Carris NW, Taylor JR GJ. Combining a GLP-1 receptor agonist and basal insulin: study evidence and practical considerations. Drugs. 2014;74(18):2141–2152. [PubMed: 25414121]
- 55.
- Yang Y, Chen S, Pan H, et al. Safety and efficiency of SGLT2 inhibitor combining with insulin in subjects with diabetes: Systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2017;96(21):e6944. doi:https://dx
.doi.org/10 .1097/MD.0000000000006944 [PMC free article: PMC5457866] [PubMed: 28538386] - 56.
- Dungan K, Merrill J, Long CBP. Effect of beta blocker use and type on hypoglycemia risk among hospitalized insulin requiring patients. Cardiovasc Diabetol. 2019;18(1):163. [PMC free article: PMC6882013] [PubMed: 31775749]
- 57.
- Zhao P, Xu P, Wan CWZ. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011;5(10). [PMC free article: PMC9040377] [PubMed: 21975743]
- 58.
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi:10.1002/hep.28431 [PubMed: 26707365] [CrossRef]
- 59.
- Leite NC, Salles GF, Araujo AL, Villela-Nogueira CACC. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29(1):113–119. doi:10.1111/j.1478-3231.2008.01718.x [PubMed: 18384521] [CrossRef]
- 60.
- Fan N, Zhang L, Xia Z, Peng L, Wang Y, Peng Y. Sex-Specific association between serum uric acid and nonalcoholic fatty liver disease in type 2 diabetic patients. J Diabetes Res. 2016;2016. doi:10.1155/2016/3805372 [PMC free article: PMC4921134] [PubMed: 27382573] [CrossRef]
- 61.
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi:10.1002/hep.29367 [PubMed: 28714183] [CrossRef]
- 62.
- Care D, Suppl SS. 4. Comprehensive medical evaluation and assessment of comorbidities: Standards of medical care in diabetes–2021. Diabetes Care. 2021;44(January):S40–S52. doi:10.2337/dc21-S004 [PubMed: 33298415] [CrossRef]
- 63.
- Singh S, Wright EEJ, Kwan AYM, et al. Glucagon-like peptide-1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab. 2017;19(2):228–238. doi:https://dx
.doi.org/10.1111/dom.12805 [PMC free article: PMC5299485] [PubMed: 27717130] - 64.
- Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486. doi:10.2337/dc10-2415 [PMC free article: PMC3120182] [PubMed: 21593294] [CrossRef]
- 65.
- Tronieri JS, Wadden TA, Chao AM, Tsai AG. Primary Care Interventions for Obesity: Review of the Evidence. Curr Obes Rep. 2019;8(2):128–136. doi:10.1007/s13679-019-00341-5 [PMC free article: PMC6520163] [PubMed: 30888632] [CrossRef]
- 66.
- Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW SBAC of SM. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sport Exerc. 2009;41(2):459–471. [PubMed: 19127177]
- 67.
- Gudzune KA, Doshi RS, Mehta AK, et al. Efficacy of commercial weight-loss programs: An updated systematic review. Ann Intern Med. 2015;162(7):501–512. doi:10.7326/M14-2238 [PMC free article: PMC4446719] [PubMed: 25844997] [CrossRef]
- 68.
- Wadden TA, Bray GAE. Handbook of Obesity Treatment, 2nd Edition. 2nd ed. Guilford Press; 2018.
- 69.
- Athinarayanan SJ, Adams RN, Hallberg SJ, McKenzie AL, Bhanpuri NH, Campbell WW, Volek JS, Phinney SD MJ. Long-Term Effects of a Novel Continuous Remote Care Intervention Including Nutritional Ketosis for the Management of Type 2 Diabetes: A 2-Year Non-randomized Clinical Trial. Front Endocrinol (Lausanne). 2019;Jun 5(10):348. [PMC free article: PMC6561315] [PubMed: 31231311]
- 70.
- Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347(October):1–16. doi:10.1136/bmj.f5934 [PMC free article: PMC3806364] [PubMed: 24149519] [CrossRef]
- 71.
- Arterburn D, Wellman R, Emiliano A, et al. Comparative effectiveness and safety of bariatric procedures for weight loss a pcornet cohort study. Ann Intern Med. 2018;169(11):741–750. doi:10.7326/M17-2786 [PMC free article: PMC6652193] [PubMed: 30383139] [CrossRef]
- 72.
- Lars Sjöström, M.D., Ph.D., Kristina Narbro, Ph.D., Sjöström C. David, M.D., Ph.D., Kristjan Karason, M.D. PD, Bo Larsson, M.D., Ph.D., Hans Wedel, Ph.D., Ted Lystig, Ph.D., Marianne Sullivan, Ph.D., Claude Bouchard PD, Björn Carlsson, M.D., Ph.D., Calle Bengtsson, M.D., Ph.D., Sven Dahlgren, M.D., Ph.D., Anders Gummesson MD, Peter Jacobson, M.D., Ph.D., Jan Karlsson, Ph.D., Anna-Karin Lindroos, Ph.D., Hans Lönroth, M.D. PD, Ingmar Näslund, M.D., Ph.D., Torsten Olbers, M.D., Ph.D., Kaj Stenlöf, M.D., Ph.D., Jarl Torgerson, M.D. PD, Göran Ågren, M.D., and Lena M.S. Carlsson, M.D., Ph.D. for the SOSS. Effects of Bariatric Surgery on Mortality in Swedish Obese Subjects. N Engl J Med. 2007;357(8):741–752. [PubMed: 17715408]
- 73.
- Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–761. doi:10.1056/NEJMoa066603 [PubMed: 17715409] [CrossRef]
- 74.
- Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr. 1997;66(2):239–246. doi:10.1093/ajcn/66.2.239 [PubMed: 9250100] [CrossRef]
- 75.
- Group ASC, Bowman L, Mafham M, et al. Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus. Sleight P Samani N, Detering E, Aubonnet P, Sandercock P, Gerstein H, Gray R, Hennekens C, Fletcher L, Achiri P, Armitage A, Bateman S, Booker V, Brown K, Butcher F, Butler E, Butler S, Cobb L, Collett A, Colmenero P, Crowther J, Fathers S, Frederick K, WP, ed. N Engl J Med. 2018;379(16):1529–1539. doi:https://dx
.doi.org/10.1056/NEJMoa1804988 [PubMed: 30146931] - 76.
- Saito Y, Okada S, Ogawa H, et al. Low-Dose Aspirin for Primary Prevention of Cardiovascular Events in Patients With Type 2 Diabetes Mellitus: 10-Year Follow-Up of a Randomized Controlled Trial. Circulation. 2017;135(7):659–670. doi:https://dx
.doi.org/10 .1161/CIRCULATIONAHA.116.025760 [PubMed: 27881565] - 77.
- Kokoska LA, Wilhelm SM, Garwood CL, Berlie HD. Aspirin for primary prevention of cardiovascular disease in patients with diabetes: A meta-analysis. Diabetes Res Clin Pract. 2016;120:31–39. doi:https://dx
.doi.org/10 .1016/j.diabres.2016.07.012 [PubMed: 27500549] - 78.
- Uchiyama S, Ishizuka N, Shimada K, et al. Aspirin for Stroke Prevention in Elderly Patients With Vascular Risk Factors: Japanese Primary Prevention Project. Stroke. 2016;47(6):1605–1611. doi:https://dx
.doi.org/10 .1161/STROKEAHA.115.012461 [PubMed: 27165949] - 79.
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2016;164(12):836–845. doi:10.7326/M16-0577 [PubMed: 27064677] [CrossRef]
- 80.
- Zinman B, Wanner C, Lachin J, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi:10.1056/nejmoa1504720 [PubMed: 26378978] [CrossRef]
- 81.
- Neal B, Perkovic V, Matthews DR, et al. Rationale, design and baseline characteristics of the CANagliflozin cardioVascular Assessment Study-Renal (CANVAS-R): A randomized, placebo-controlled trial. Diabetes Obes Metab. 2017;19(3):387–393. doi:https://dx
.doi.org/10.1111/dom.12829 [PMC free article: PMC5348724] [PubMed: 28120497] - 82.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. Bergenstal R Daniels G, Mann J, Marso SP, Moses AC, Nauck M, Nissen S, Pocock S, Poulter N, Steinberg W, Zinman B, Frandsen KB, Stockner M, Kristensen P, Ravn LS, Zychma M, Abell S, Davis T, D’Emden M, Ding SA, Gilfillan C, Greenaway T, Gunawan F, Ho J, BJ, ed. N Engl J Med. 2016;375(4):311–322. doi:https://dx
.doi.org/10.1056/NEJMoa1603827 [PMC free article: PMC4985288] [PubMed: 27295427] - 83.
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. Fitchett D Boon N, Gardner T, Inzucchi S, Chaitman BR, Bach R, Gosselin G, Laddu AA, Albert SG, Gyawali CP, Mullady DK, Block G, Bushinsky D, Martin K, Alsheklhee A, Cruz-Flores S, Feen ES, Jacoby MA, Picus J, Schroeder MA, Akduman L, Dudney BW, Aberkane BJD, ed. N Engl J Med. 2016;375(19):1834–1844. [PubMed: 27633186]
- 84.
- Husain M, Birkenfeld AL, Donsmark M, et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2019;381(9):841–851. doi:10.1056/nejmoa1901118 [PubMed: 31185157] [CrossRef]
- 85.
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130. doi:10.1016/S0140-6736(19)31149-3 [PubMed: 31189511] [CrossRef]
- 86.
- Young LH, Wackers FJT, Chyun DA et al. Cardiac Outcomes After Screening for Asymptomatic Coronary Artery Disease in Patients With Type 2 Diabetes: The DIAD Study: A Randomized Controlled Trial. JAMA. 2009;301(15):1547–1555. doi:10.1001/jama.2009.476 [PMC free article: PMC2895332] [PubMed: 19366774] [CrossRef]
- 87.
- Curtis JM, Horton ES, Bahnson J, et al. Prevalence and predictors of abnormal cardiovascular responses to exercise testing among individuals with type 2 diabetes: The look ahead (action for health in diabetes) study. Diabetes Care. 2010;33(4):901–907. doi:10.2337/dc09-1787 [PMC free article: PMC2845049] [PubMed: 20056948] [CrossRef]
- 88.
- Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115(3):387–397. doi:10.1161/CIRCULATIONAHA.106.634949 [PubMed: 17242296] [CrossRef]
- 89.
- Bae JH, Park EG, Kim S, Kim SG, Hahn S, Kim NH. Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Renal Outcomes in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sci Rep. 2019;9(1):1–9. doi:10.1038/s41598-019-49525-y [PMC free article: PMC6736944] [PubMed: 31506585] [CrossRef]
- 90.
- Pecoraro RE, Reiber GE BE. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13(5):513–521. doi:10.2337/diacare.13.5.513 [PubMed: 2351029] [CrossRef]
- 91.
- Bally M, Dendukuri N, Rich B, et al. Risk of acute myocardial infarction with NSAIDs in real world use: Bayesian meta-analysis of individual patient data. BMJ. 2017;357(Cox 2). doi:10.1136/bmj.j1909 [PMC free article: PMC5423546] [PubMed: 28487435] [CrossRef]
- 92.
- Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(SUPPL. 2). doi:10.2337/dc07-s225 [PubMed: 17596481] [CrossRef]
- 93.
- Hilary A. Roeder, Thomas R. Moore GAR. Changes in Postpartum Insulin Requirements for Patients with Well-Controlled Type 1 Diabetes. Am J Perinatol. 2016;33(7):683–687. doi:10.1055/s-0036-1571323 [PubMed: 26862721] [CrossRef]
- 94.
- Guerin, A, Nisenbaum, R, Ray J. Use of Maternal GHb Concentration to Estimate the Risk of Congenital Anomalies. Diabetes Care. 2007;30(7):1920–1925. doi:10.2337/dc07-0278.Additional [PubMed: 17446531] [CrossRef]
- 95.
- Jensen DM, Korsholm L, Ovesen P, et al. Peri-conceptional A1C and risk of serious adverse pregnancy outcome in 933 women with type 1 diabetes. Diabetes Care. 2009;32(6):1046–1048. doi:10.2337/dc08-2061 [PMC free article: PMC2681038] [PubMed: 19265024] [CrossRef]
- 96.
- Nielsen GL, M⊘ller M, S⊘rensen HT. HbA1c in early diabetic pregnancy and pregnancy outcomes: A Danish population-based cohort study of 573 pregnancies in women with type 1 diabetes. Diabetes Care. 2006;29(12):2612–2616. doi:10.2337/dc06-0914 [PubMed: 17130193] [CrossRef]
- 97.
- Suhonen L, Hiilesmaa V, Teramo K. Glycaemic control during early pregnancy and fetal malformations in women with type I diabetes mellitus. Diabetologia. 2000;43(1):79–82. doi:10.1007/s001250050010 [PubMed: 10663219] [CrossRef]
- 98.
- Jovanovič, L., Liang, Y., Weng, W., Hamilton, M., Chen, L., and Wintfeld N. Trends in the incidence of diabetes, its clinical sequelae, and associated costs in pregnancy. Diabetes Metab Res Rev,. 2015;31:707–716. doi:10.1002/dmrr.2656 [PMC free article: PMC4676929] [PubMed: 25899622] [CrossRef]
- 99.
- Manderson JG, Patterson CC, Hadden DR, Traub AI, Ennis C MD. Preprandial versus postprandial blood glucose monitoring in type 1 diabetic pregnancy: a randomized controlled clinical trial. Am J Obs Gynecol. 2003;189(2):507–512. doi:10.1067/s0002-9378(03)00497-6 [PubMed: 14520226] [CrossRef]
- 100.
- de Veciana M, Major CA, Morgan MA et al. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995;333(19):1237–1241. [PubMed: 7565999]
- 101.
- Jovanovic-Peterson L, Peterson CM, Reed GF et al. Maternal postprandial glucose levels and infant birth weight: the Diabetes in Early Pregnancy Study. The National Institute of Child Health and Human Development--Diabetes in Early Pregnancy Study. Am J Obs Gyneco. 1991;164(1):103–111. [PubMed: 1986596]
- 102.
- Feig DS, Corcoy R, Donovan LE et al. Pumps or Multiple Daily Injections in Pregnancy Involving Type 1 Diabetes: A Prespecified Analysis of the CONCEPTT Randomized Trial. Diabetes Care. 2018;41(12):2471–2479. doi:10.2337/dc18-1437 [PubMed: 30327362] [CrossRef]
- 103.
- &NA; Intensive Blood-Glucose Control with Sulfonylureas or Insulin Compared with Conventional Treatment and Risk of Complications in Patients with Type 2 Diabetes. Endocrinologist. 1999;9(2):149. doi:10.1097/00019616-199903000-00016 [CrossRef]
- 104.
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854–865. doi:10.1016/S0140-6736(98)07037-8 [PubMed: 9742977] [CrossRef]
- 105.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. N Engl J Med. 2008;359(15):1577–1589. doi:10.1056/nejmoa0806470 [PubMed: 18784090] [CrossRef]
- 106.
- Oetjen E. Long-Term Effects of Intensive Glucose Lowering on Cardiovascular Outcomes. Yearb Med. 2012;2012:415–416. doi:10.1016/s0084-3873(12)00310-0 [CrossRef]
- 107.
- Siraj ES, Rubin DJ, Riddle MC, et al. Insulin dose and cardiovascular mortality in the ACCORD trial. Diabetes Care. 2015;38(11):2000–2008. doi:10.2337/dc15-0598 [PMC free article: PMC4876773] [PubMed: 26464212] [CrossRef]
- 108.
- Riddle MC. Effects of intensive glucose lowering in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation. 2010;122(8):844–846. doi:10.1161/CIRCULATIONAHA.110.960138 [PMC free article: PMC2980502] [PubMed: 20733112] [CrossRef]
- 109.
- Papademetriou V, Lovato L, Doumas M, et al. Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int. 2015;87(3):649–659. doi:10.1038/ki.2014.296 [PubMed: 25229335] [CrossRef]
- 110.
- Basu S, Sussman JB, Berkowitz SA, et al. Validation of Risk Equations for Complications of Type 2 Diabetes (RECODe) Using Individual Participant Data From Diverse Longitudinal Cohorts in the U.S. Diabetes Care. 2018;41(3):586–595. doi:https://dx
.doi.org/10.2337/dc17-2002 [PMC free article: PMC5829967] [PubMed: 29269511] - 111.
- Group A to CCR in DF-O (ACCORDION) ESG and the A to CCR in DF-O (ACCORDION) S. Persistent Effects of Intensive Glycemic Control on Retinopathy in Type 2 Diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Follow-On Study. Diabetes Care. 2016;39(7):1089–1100. doi:https://dx
.doi.org/10.2337/dc16-0024 [PMC free article: PMC4915557] [PubMed: 27289122] - 112.
- The ADVANCE Collaborative Group. Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2008;358:2560–2572. [PubMed: 18539916]
- 113.
- Zoungas S, Chalmers J, Ninomiya T, et al. Association of HbA 1c levels with vascular complications and death in patients with type 2 diabetes: Evidence of glycaemic thresholds. Diabetologia. 2012;55(3):636–643. doi:10.1007/s00125-011-2404-1 [PubMed: 22186981] [CrossRef]
- 114.
- Of D, Mellitus D. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(SUPPL.1):81–90. doi:10.2337/dc14-S081 [PubMed: 24357215] [CrossRef]
- 115.
- Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of Glycemic Control and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;372(23):2197–2206. doi:10.1056/nejmoa1414266 [PubMed: 26039600] [CrossRef]
- 116.
- Agrawal L, Azad N, Bahn GD, et al. Long-term follow-up of intensive glycaemic control on renal outcomes in the Veterans Affairs Diabetes Trial (VADT). Diabetologia. 2018;61(2):295–299. doi:https://dx
.doi.org/10 .1007/s00125-017-4473-2 [PMC free article: PMC5747983] [PubMed: 29101421]
APPROVALS
| P&T | Date: 6/16/2021 |
| ACOC | Date: 7/22/2021 |
| CPC | Date: 9/28/2021 |
| ECCA | Date: 10/26/2021 |
Appendix A. Four Large Clinical Trials Evaluating Targets for Therapy of Type 2 Diabetes
Targets for therapy of Type 2 diabetes have been evaluated in four large clinical trials: UK Prospective Diabetes Study (UKPDS), Action to Control Cardiovascular Risk in Diabetes study (ACCORD), Action in Diabetes and Vascular Disease Controlled Evaluation (ADVANCE) and The VA Diabetes Trial (VADT).
UKPDS
The UKPDS randomized 3687 subjects newly diagnosed with Type 2 diabetes (mean age 54 years) without significant macrovascular or renal disease to intense control (FPG<108 mg/dL) with either sulfonylurea or insulin compared to conventional control (FPG<270mg/dl) over 10 years. Mean A1c achieved was 7% in the intervention arm and 7.9% in the conventional arm. Those in the sulfonylurea/insulin intervention arm had a 12% lower diabetes complication composite endpoint (p=0.029) (driven largely by the reduction in the need for retinal photocoagulation).103 The UKPDS also included a metformin intervention arm, where the achieved A1c in the intensive arm was 7.4 % compared to 8% in the conventional arm. Compared with the conventional treatment, those in the intensive metformin arm had a reduction of 32% (95% CI 13–47, p=0·002) for any diabetes-related endpoint, 42% for diabetes-related death (9–63, p=0·017), and 36% for all-cause mortality (9–55, p=0·011), all significantly greater reductions than in the sulfonylurea/insulin arm.104 One year after the conclusion of the UKPDS trial, there was no difference in glycemic control found between the groups. However, ten years after the end of the UKPDS trial, between group differences persisted. In the sulfonylurea–insulin group, relative reductions in risk for any diabetes-related end point (9%, P = 0.04) and microvascular disease (24%, P = 0.001) persisted, and risk reductions emerged for myocardial infarction (15%, P = 0.01) and death from any cause (13%, P = 0.007). In the metformin arm, risk reductions persisted for any diabetes-related end point (21%, P = 0.01), myocardial infarction (33%, P = 0.005), and death from any cause (27%, P = 0.002).105
ACCORD
ACCORD recruited 10,109 subjects between the ages of 40 and 79 with Type 2 diabetes and either known cardiovascular disease or known risk factors for cardiovascular disease and randomized them to A1c targets <6% (achieved 6.4%) or 7-7.9% (achieved 7.5%). No standard treatment regimen was applied. The study was terminated at 3.7 years due to increased all-cause mortality (hazard ratio, 1.21; 95% confidence interval [CI], 1.02 to 1.44y) in the intense arm.106 This finding was surprising and multiple post hoc analyses have tried to understand it. As no defined medication treatment protocol was used in ACCORD, one avenue of investigation was that perhaps a specific medication or class of medications used more frequently in the intense arm increased mortality. However, that did not appear to be the case when studied. There was no relationship between insulin use/dose for example.107 Another assumption that hypoglycemia was the cause of increased mortality did not appear to be correct. Increased mortality in the intensive arm was observed in subjects with an average A1c of >7 and either no change or an increase in A1c in the 1st year of the trial.108 Analysis did show that DKD,109 increased BMI, and increased age110 were associated with increased mortality in the intensive control group. Despite increased all-cause mortality in the intense arm, ACCORD did demonstrate a benefit of acute control through a reduction in the progression of retinopathy -5.8% in the intense arm versus 12.7% in the standard arm (adjusted odds ratio [aOR] 0.42, 95% CI 0.28–0.63, P <0.0001).111
ADVANCE
ADVANCE recruited 11,140 patients with Type 2 diabetes who were 55 years of age or older with at least one CV risk factor or a known macro or microvascular complication of diabetes. Subjects were randomized to intense control (target HbA1c of <6.5% and achieved A1c of 6.53%_versus standard (no set target HbA1c and achieved HbA1c of 7.3 %). Subjects received gliclazide plus other medications (metformin, thiazolinedione, acarbose, insulin) as needed in a sequential manner to achieve goal, and followed for 5 years. ADVANCE found a 10% relative risk reduction for the combined outcome of major macrovascular and microvascular outcomes. (18.1% vs 20.0% HR 0,90; 85% CI 0.82-0.98, P=0.01) This finding was driven primarily by a reduction in renal events.112 One particularly intriguing analysis of ADVANCE data suggested that there were A1c thresholds. At HbA1c levels below 7.0% for macrovascular events and death, and below 6.5% for microvascular events, there was no significant change in risks, but that above these thresholds, the risks increased. For every 1% higher HbA1c level, there was a 38% higher risk of a macrovascular event, a 40% higher risk of a microvascular event and a 38% higher risk of death (all p<0.0001).113 Another interesting analysis from ADVANCE demonstrated an increase in both macrovascular and microvascular risk with visit to visit variability in A1c and fasting glucose.114
VATD
The VADT study recruited veterans (mean age 60.4 years, mean duration of diabetes 11.4 years, mean A1c 9.4%), and randomized to tight control (achieved A1c of 6.9% versus 8.4%). A reduction in cardiovascular events was seen 5 years after the end of the VADT in the intense group. (Hazard ratio, 0.83; 95% confidence interval [CI], 0.70 to 0.99; P = 0.04)115 as were persistent renal benefits as more of those in the intensive arm had an eGFR >60 mL min–1 1.73 m–2 (OR 1.34 [95% CI 1.05, 1.71], p = 0.02).116
A metanalysis of these studies, which included 27,049 participants, found that compared with less intensive glucose control, more intensive glucose control resulted in a reduction of relative risk by 20% for kidney end points (hazard ratio 0·80, 95% CI 0·72 to 0·88; p<0·0001) and by 13% for eye points (0·87, 0·76 to 1·00; p=0·04). 116
Appendix B. Insulin Initiation and Adjustment Protocol
- Start with NPH, detemir, glargine, or degludec
- The choice may vary depending on concerns regarding endogenous insulin secretion, need for meal- time insulin coverage, cost and convenience.
- All patients started on insulin should demonstrate use of a glucometer and be educated on recognition and treatment of hypoglycemia.Glargine, degludec, detemir, or NPH once dailyOf note: NPH and detemir do not last for 24 hours and usually require twice daily dosing
- Continue metformin +/- other antihyperglycemic agents depending on preprandial glucose.
- Add 10-20 units of glargine, degludec, detemir or NPH insulin daily
- Then increase insulin by 10-20% or 2-4 units every 3 days until attaining fasting blood glucose goal without hypoglycemia.
- Consider adding either rapid or regular insulin before meals if post-prandial glucose >180 mg/dL.
NPH or detemir insulin twice daily- Continue metformin +/- other antihyperglycemic agents as appropriate
- Add 5-10 units of NPH or detemir insulin at breakfast and dinner (or bedtime).
- Then increase insulin by 10-20% or at least 2 units every 3 days until attaining a fasting blood glucose and pre-dinner glucose goal without hypoglycemia.
- Consider adding either rapid or regular insulin before meals if post-prandial glucose >180 mg/dL.
Premixed insulin (intermediate or long & short-acting or rapid-acting mixtures)- Continue metformin, discontinue sulfonylurea.
- Add 10 units of pre-mixed insulin at breakfast and dinner.
- Then increase pre-breakfast and/or pre-dinner insulin by 10-20% or at least 2 units every 3 days until attaining a fasting and pre-meal glucose goal without hypoglycemia.
Insulin Adjustment Protocol
| If overnight or before breakfast glucoses are above/below target | Adjust the supper or bedtime dose of NPH, detemir, glargine or degludec |
| If before lunch glucoses are above/below target | Adjust the breakfast dose of regular, rapid acting or ultra-rapid acting insulin |
| If before supper glucoses are above/below target | Adjust the breakfast dose of NPH OR the lunch dose of regular, rapid acting or ultra-rapid acting insulin |
| If before bedtime glucoses are above/below target | Adjust the supper dose of regular, rapid acting or ultra-rapid acting insulin |
If fasting glucose levels are significantly higher than bedtime levels (i.e. twice as high), consider nocturnal hypoglycemia. Have the patient check glucose level around 3 AM for 2 days during the week. If glucose levels are:
| Increase the NPH supper dose Decrease the NPH supper dose |
Insulin adjustment for patients
| For NPH bedtime or Lantus dosing: | |
|---|---|
| 3 consecutive morning readings >130 | increase bedtime NPH or Lantus by 2 units |
| 3 consecutive morning readings >150 | increase bedtime NPH or Lantus by 4 units |
| For NPH twice a day: | |
| 3 consecutive morning readings >130 | increase evening NPH by 2 units |
| 3 consecutive morning readings >150 | increase evening NPH by 4 units |
| 3 consecutive evening readings >130 | increase morning NPH by 2 units |
| 3 consecutive evening readings >150 | increase morning NPH by 4 units |
These guidelines should not be construed as including all proper methods of care or excluding other acceptable methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding any specific clinical procedure or treatment must be made by the physician in light of the circumstances presented by the patient.
Data Availability
These links to Internal UMHS Guidelines contain proprietary information so are only accessible to appropriate Michigan Medicine staff. For more information, contact the authors or publisher.
Supplementary material can be found at http://www.uofmhealth.org/provider/clinical-care-guidelines
Internal UMHS Guidelines contain proprietary information so are only accessible to appropriate Michigan Medicine staff. For more information, contact the authors or publisher.
Created: May 1996; Last Update: October 2021.
- Clinical Problem: Prevalence and Outcomes
- Complementary and Alternative Therapies
- Special Populations
- Older Individuals with Diabetes
- Coordination with Other Care Providers
- Related National Guidelines and Performance Measures
- Guideline Development Methodology
- Acknowledgments
- References
- APPROVALS
- Four Large Clinical Trials Evaluating Targets for Therapy of Type 2 Diabetes
- Insulin Initiation and Adjustment Protocol
- NLM CatalogRelated NLM Catalog Entries
- PMCPubMed Central citations
- PubMedLinks to PubMed
- Review Interventions for improving adherence to treatment recommendations in people with type 2 diabetes mellitus.[Cochrane Database Syst Rev. 2005]Review Interventions for improving adherence to treatment recommendations in people with type 2 diabetes mellitus.Vermeire E, Wens J, Van Royen P, Biot Y, Hearnshaw H, Lindenmeyer A. Cochrane Database Syst Rev. 2005 Apr 18; 2005(2):CD003638. Epub 2005 Apr 18.
- Review Adherence To Diabetes Mellitus Treatment Guidelines From Theory To Practice: The Missing Link.[J Ayub Med Coll Abbottabad. 2016]Review Adherence To Diabetes Mellitus Treatment Guidelines From Theory To Practice: The Missing Link.Hashmi NR, Khan SA. J Ayub Med Coll Abbottabad. 2016 Oct-Dec; 28(4):802-808.
- Review [Preventing type 2 diabetes complications: from evidence-based recommendations to patient-based reality].[Rev Med Suisse. 2005]Review [Preventing type 2 diabetes complications: from evidence-based recommendations to patient-based reality].Beer S, Kunz GA, Ruiz J. Rev Med Suisse. 2005 Jun 1; 1(22):1492-8.
- Review Kidney Disease in Diabetes.[Diabetes in America. 2018]Review Kidney Disease in Diabetes.Pavkov ME, Collins AJ, Coresh J, Nelson RG. Diabetes in America. 2018 Aug
- The 2021-2022 position of Brazilian Diabetes Society on diabetic kidney disease (DKD) management: an evidence-based guideline to clinical practice. Screening and treatment of hyperglycemia, arterial hypertension, and dyslipidemia in the patient with DKD.[Diabetol Metab Syndr. 2022]The 2021-2022 position of Brazilian Diabetes Society on diabetic kidney disease (DKD) management: an evidence-based guideline to clinical practice. Screening and treatment of hyperglycemia, arterial hypertension, and dyslipidemia in the patient with DKD.de Sá JR, Rangel EB, Canani LH, Bauer AC, Escott GM, Zelmanovitz T, Bertoluci MC, Silveiro SP. Diabetol Metab Syndr. 2022 Jun 11; 14(1):81. Epub 2022 Jun 11.
- Management of Type 2 Diabetes MellitusManagement of Type 2 Diabetes Mellitus
Your browsing activity is empty.
Activity recording is turned off.
See more...