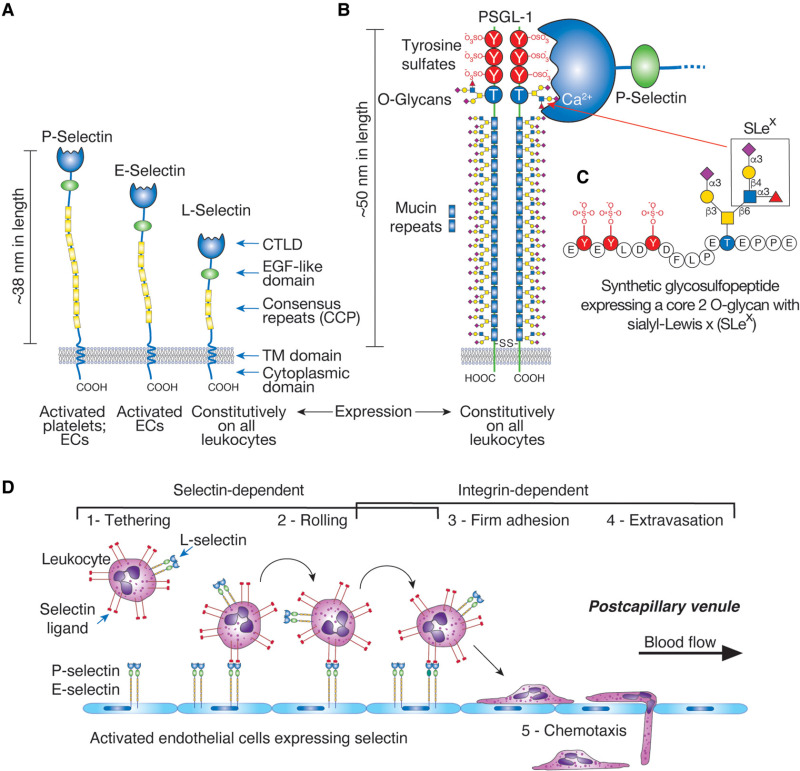
Structures and functions of selectins. (A) Overall domain structures of P-selectin, E-selectin, and L-selectin and their expression patterns are indicated. P-selectin also forms homodimers in the membrane. (The key for the domain structures is shown in Figure .) (B) The predicted disulfide-bonded dimeric form of PSGL-1 on leukocytes is shown. PSGL-1 has three potential sites for N-glycosylation and, as indicated, multiple sites for O-glycosylation. The sulfated tyrosine residues at the extreme amino terminus are indicated. The Ca++-dependent interaction between P-selectin and PSGL-1 through their amino-terminal domains is indicated. L-selectin also binds to the same region of PSGL-1, although with different kinetics and affinity. (C) The major fucose-containing core-2 O-glycan identified in PSGL-1 is required for binding to selectins and is shown on a synthetic GSP generated based on the amino-terminal sequence of human PSGL-1. The sialyl-Lewis x determinant on a core 2 O-glycan is boxed. E-selectin may bind to more distal glycans expressing the SLex determinant. (D) Tethering of circulating leukocytes to activated endothelium via interactions between selectins and their ligands. In normal venules, leukocytes flow without adhesive interactions with the endothelium, but in inflamed vessels, selectins and integrin ligands are expressed on endothelial surfaces. This leads to tethering, rolling, and arrest of circulating leukocytes and their eventual extravasation from the circulation to the surrounding tissue. Selectin-dependent interactions also occur through E-selectin binding to glycans on leukocyte CD44 (HCELL). When adherent cells become activated by regionally presented chemokines or lipid autacoids, the activated leukocytes express integrins (e.g., LFA-1, CD11a/CD18 and Mac-1, CD11b/CD18) that interact with immunoglobulin-like counterreceptors on endothelial cells (ICAM-1, ICAM-2) to strengthen the adhesion and promote the transmigration of cells from the circulation into the underlying tissues. P-selectin is normally expressed in Weibel–Palade bodies of endothelial cells, but within minutes after endothelial cell activation by thrombin, histamine, hypoxia, or injury, these bodies fuse with the plasma membrane, promoting the expression of P-selectin on the endothelial cell surface. Similarly, P-selectin stored in the α-granules of platelets becomes expressed on the surfaces of platelets within minutes after platelet activation. E-selectin is expressed by activated endothelial cells through transcription-dependent pathways and is inducible by TNFα, IL1β, and LPS, mediated in part through NF-κB-dependent events. E-selectin cooperates with P- and L-selectin to recruit leukocytes to sites of inflammation.