Summary
Clinical characteristics.
Long-chain hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency and trifunctional protein (TFP) deficiency are caused by impairment of mitochondrial TFP. TFP has three enzymatic activities – long-chain enoyl-CoA hydratase, long-chain 3-hydroxyacyl-CoA dehydrogenase, and long-chain 3-ketoacyl-CoA thiolase. In individuals with LCHAD deficiency, there is isolated deficiency of long-chain 3-hydroxyacyl-CoA dehydrogenase, while deficiency of all three enzymes occurs in individuals with TFP deficiency.
Individuals with TFP deficiency can present with a severe-to-mild phenotype, while individuals with LCHAD deficiency typically present with a severe-to-intermediate phenotype.
Neonates with the severe phenotype present within a few days of birth with hypoglycemia, hepatomegaly, encephalopathy, and often cardiomyopathy.
The intermediate phenotype is characterized by hypoketotic hypoglycemia precipitated by infection or fasting in infancy.
The mild (late-onset) phenotype is characterized by myopathy and/or neuropathy.
Long-term complications include peripheral neuropathy and retinopathy.
Diagnosis/testing.
The diagnosis of LCHAD/TFP deficiency is established in a proband with elevation of long-chain 3-hydroxyacylcarnitine species in plasma and/or increased excretion of 3-hydroxy-dicarboxylic acids in urine in combination with identification of biallelic pathogenic variants in HADHA or HADHB by molecular genetic testing.
Distinguishing LCHAD deficiency from TFP deficiency requires identification of isolated long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency on enzymatic assay in lymphocytes or skin fibroblasts. TFP deficiency is confirmed by the identification of deficiencies in all three TFP enzymatic activities (long-chain enoyl-CoA hydratase, long-chain 3-hydroxyacyl-CoA dehydrogenase, and long-chain 3-ketoacyl-CoA thiolase) in lymphocytes or skin fibroblasts.
Management.
Treatment: Avoidance of fasting using frequent feeds, decreasing feeding intervals and supplemental carbohydrates during illness, and continuing overnight feeds in older children as needed for hypoglycemia; medium-chain triglyceride (MCT) or triheptanoin supplementation; low-fat diet; carnitine supplementation in those with carnitine deficiency; feeding therapy and gastrostomy tube as needed; developmental services; and treatment of cardiac dysfunction, peripheral neuropathy, and retinopathy by relevant specialists. Emergency outpatient treatment for mild decompensation includes decreasing the fasting interval, administration of antipyretics for fever, and antiemetics as needed for vomiting. Acute treatment includes hospitalization with intravenous fluid containing at least 10% dextrose, and bicarbonate therapy for severe metabolic acidosis; management of hyperammonemia and rhabdomyolysis; and management of cardiomyopathy per cardiologist.
Prevention of primary manifestations: Avoidance of fasting; supplementation with MCT or triheptanoin; strict dietary management; education of parents and caregivers to ensure prompt treatment; written protocol for emergency treatment.
Surveillance: Monitor nutrition, serum plasma free and total carnitine, acylcarnitine profile, creatine kinase, AST, and ALT with frequency based on age; annual comprehensive fatty acid profile; monitor head size, growth, and development at each visit throughout childhood; neuropsychological testing and quality of life assessments as needed; EKG and echocardiography annually or more frequently as needed; annual neurology evaluation with nerve conduction velocity and electromyography as needed; annual ophthalmology evaluation with electroretinography every two to three years.
Agents/circumstances to avoid: Fasting; inadequate calories during stressors; dehydration; high-fat diets including ketogenic and carbohydrate restricted diet; anesthetics that contain high doses of long-chain fatty acids; intravenous intralipids during acute metabolic crisis.
Evaluation of relatives at risk: Testing of all at-risk sibs of any age is warranted (targeted molecular genetic testing if the familial pathogenic variants are known or plasma acylcarnitine profile, plasma free and total carnitine, and urine organic acid assay if the pathogenic variants in the family are not known) to allow for early diagnosis and treatment of LCHAD/TFP deficiency.
Pregnancy management: Increase MCT intake in the third trimester; high dextrose infusion in the peripartum period. Monitor for HELLP syndrome and acute fatty liver of pregnancy in pregnant females who are heterozygous for an HADHA or HADHB pathogenic variant (including suspected carriers).
Genetic counseling.
LCHAD/TFP deficiency is inherited in an autosomal recessive manner. If both parents are known to be heterozygous for an HADHA or HADHB pathogenic variant, each sib of an affected individual has at conception a 25% chance of being affected, a 50% chance of being an asymptomatic carrier, and a 25% chance of inheriting neither of the familial pathogenic variants. Once the HADHA or HADHB pathogenic variants have been identified in an affected family member, carrier testing for at-risk relatives and prenatal and preimplantation genetic testing are possible.
Diagnosis
No consensus clinical diagnostic criteria for long-chain hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency or trifunctional protein (TFP) deficiency have been published.
Suggestive Findings
Scenario 1: Abnormal Newborn Screening (NBS) Result
NBS for LCHAD/TFP deficiency is primarily based on quantification of the analytes 3-hydroxypalmitoyl carnitine (C16-OH) and 3-hydroxyoleoylcarnitine (C18:1-OH) on dried blood spots.
C16-OH and C18:1-OH values above the cutoff reported by the screening laboratory are considered positive and require follow-up biochemical testing including plasma acylcarnitine and urine organic acid profiles.
If the follow-up biochemical testing supports the likelihood of LCHAD/TFP deficiency, additional testing is required to establish the diagnosis (see Establishing the Diagnosis).
The following medical interventions need to begin immediately on receipt of an abnormal NBS result while additional testing is performed to determine whether this is a true positive NBS result and to establish a definitive diagnosis of LCHAD/TFP deficiency:
Evaluation of the newborn to ascertain clinical status
Education of the caregivers to avoid prolonged fasting and to monitor for decreased oral intake, vomiting, or lethargy
Immediate intervention (to be considered if the newborn is not doing well clinically) possibly including admission to the hospital, fluid resuscitation, infusion of IV dextrose (10% or higher), and cardiac evaluation
Scenario 2: Symptomatic Individual
Supportive – but nonspecific – clinical findings, laboratory findings, and family history include the following.
Clinical findings
Supportive laboratory findings
Nonspecific:
Hypoglycemia (nonketotic or hypoketotic) with blood glucose often <45 mg/dL
Urinalysis that demonstrates the absence of ketones in the setting of hypoglycemia
Metabolic acidosis
Lactic acidosis
Hyperammonemia: blood ammonia level may be >200 µmol/L in newborns and >100 µmol/L after the neonatal period
Elevated liver transaminases (AST, ALT)
Elevated creatine kinase (CK), particularly in the late-onset myopathic form. A CK value greater than five times the upper limit of reference is suggestive of rhabdomyolysis (range 1,000-100,000 IU/L). A CK value of >15,000 IU/L at presentation increases the risk for acute kidney injury [
Bosch et al 2009].
Specific:
Plasma acylcarnitine profile. The elevation of 3-hydroxy derivatives of C16, C18, and C18:1 is highly suggestive of LCHAD/TFP deficiency. The plasma acylcarnitine profile typically shows elevations of C16-OH, C18-OH, C18:1-OH, and elevated ratios of C16-OH/C16 and C18-OH/C18.
Urine organic acid analysis. Elevations of 3-hydroxy-dicarboxylic acids and lactic acid
Note: Because elevations of these metabolites can be intermittent particularly in individuals with milder disease, follow-up testing is required to establish the diagnosis of LCHAD/TFP deficiency (see Establishing the Diagnosis) [Elizondo et al 2020].
Family history is consistent with autosomal recessive inheritance (e.g., affected sibs and/or parental consanguinity). Absence of a known family history does not preclude the diagnosis.
Establishing the Diagnosis
The diagnosis of LCHAD deficiency is established in a proband with elevation of long-chain 3-hydroxyacylcarnitine species in plasma and/or increased excretion of 3-hydroxy-dicarboxylic acids in urine in combination with identification of biallelic pathogenic (or likely pathogenic) variants in HADHA by molecular genetic testing (see Table 1). Distinguishing LCHAD deficiency from TFP deficiency requires identification of isolated long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency on enzymatic assay in lymphocytes or skin fibroblasts.
The diagnosis of TFP deficiency is established in a proband with elevation of long-chain 3-hydroxyacylcarnitine species in plasma and/or increased excretion of 3-hydroxy-dicarboxylic acids in urine in combination with identification of biallelic pathogenic (or likely pathogenic) variants in HADHA or HADHB by molecular genetic testing (see Table 1). Distinguishing TFP deficiency from LCHAD deficiency requires identification of deficiency in all three TFP enzymatic activities (long-chain enoyl-CoA hydratase, long-chain 3-hydroxyacyl-CoA dehydrogenase, and long-chain 3-ketoacyl-CoA thiolase) in lymphocytes or skin fibroblasts.
Note: (1) Per ACMG/AMP variant interpretation guidelines, the terms "pathogenic variants" and "likely pathogenic variants" are synonymous in a clinical setting, meaning that both are considered diagnostic and both can be used for clinical decision making [Richards et al 2015]. Reference to "pathogenic variants" in this section is understood to include any likely pathogenic variants. (2) Most affected individuals have an abnormal acylcarnitine profile. An individual with persistent abnormal acylcarnitine profile is presumed to have LCHAD/TFP deficiency even if only one pathogenic variant is identified.
Molecular Genetic Testing Approaches
Scenario 1: Abnormal newborn screening (NBS) result. When NBS results and other laboratory findings suggest the diagnosis of LCHAD/TFP deficiency, molecular genetic testing approaches can include single-gene testing or use of a multigene panel.
Serial single-gene testing. Sequence analysis detects small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected.
Perform sequence analysis of HADHA first. If only one pathogenic variant is found, perform gene-targeted deletion/duplication analysis to detect intragenic deletions or duplications.
If HADHA testing is negative, perform sequence analysis of HADHB. If only one pathogenic variant is found, perform gene-targeted deletion/duplication analysis to detect intragenic deletions or duplications.
A multigene panel that includes
HADHA,
HADHB, and other genes of interest (see
Differential Diagnosis) may be considered to identify the genetic cause of the condition while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. Note: (1) The genes included in the panel and the diagnostic sensitivity of the testing used for each gene vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this
GeneReview. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests.
For an introduction to multigene panels click
here. More detailed information for clinicians ordering genetic tests can be found
here.
Scenario 2: Symptomatic individual. For a symptomatic individual who has findings associated with late-onset TFP deficiency OR neonatal-onset LCHAD/TFP deficiency that has not been treated (because symptoms occurred before NBS results were returned, NBS was not performed, or NBS yielded a false negative result), molecular genetic testing approaches can include serial single-gene testing or use of a multigene panel.
When the diagnosis of LCHAD/TFP deficiency has not been considered, comprehensive genomic testing, which does not require the clinician to determine which gene(s) are likely involved, is an option. Exome sequencing is most commonly used; genome sequencing is also possible.
For an introduction to comprehensive genomic testing click here. More detailed information for clinicians ordering genomic testing can be found here.
Table 1.
Molecular Genetic Testing Used in LCHAD/TFP Deficiency
View in own window
| Gene 1, 2 | Proportion of LCHAD/TFP Deficiency Attributed to Pathogenic Variants in Gene | Proportion of Pathogenic Variants 3 Detectable by Method |
|---|
| Sequence analysis 4 | Gene-targeted deletion/duplication analysis 5 |
|---|
|
HADHA
| 100% (LCHAD)
~50% (TFP) | <100% 6 | 2 persons w/TFP deficiency 7 |
|
HADHB
| ~50% (TFP) | <100% 6 | ≥2 persons w/TFP deficiency 8 |
LCHAD = long-chain hydroxyacyl-CoA dehydrogenase; TFP = trifunctional protein
- 1.
Genes are listed in alphabetic order.
- 2.
- 3.
- 4.
Sequence analysis detects variants that are benign, likely benign, of uncertain significance, likely pathogenic, or pathogenic. Variants may include small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected. For issues to consider in interpretation of sequence analysis results, click here.
- 5.
Gene-targeted deletion/duplication analysis detects intragenic deletions or duplications. Methods used may include a range of techniques such as quantitative PCR, long-range PCR, multiplex ligation-dependent probe amplification (MLPA), and a gene-targeted microarray designed to detect single-exon deletions or duplications.
- 6.
Data derived from the subscription-based professional view of Human Gene Mutation Database [Stenson et al 2020]
- 7.
To date, large deletions and/or duplications have not been reported in individuals with LCHAD deficiency. Two large HADHA deletions have been reported in individuals with TFP deficiency [Djouadi et al 2016, Bo et al 2017].
- 8.
Two large HADHB deletions have been reported in individuals with TFP deficiency [Boutron et al 2011, Aradhya et al 2012]. A third individual with neonatal-onset cardiomyopathy [Wang et al 2012] was reported; the biochemical profile was not described, but this individual likely had TFP deficiency.
Biochemical Testing Approaches
In vitro probe analysis. Skin fibroblasts incubated with palmitic acid and culture medium can be assayed for acylcarnitine after 96 hours of incubation. In individuals with LCHAD/TFP deficiency there is substantial accumulation of C16-OH [Okun et al 2002].
Clinical Characteristics
Clinical Description
Long-chain hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency and trifunctional protein (TFP) deficiency are caused by impairment of mitochondrial TFP. TFP has three enzymatic activities – long-chain enoyl-CoA hydratase, long-chain 3-hydroxyacyl-CoA dehydrogenase, and long-chain 3-ketoacyl-CoA thiolase. Deficiency of the enzyme long-chain 3-hydroxyacyl-CoA dehydrogenase occurs in individuals with LCHAD deficiency, while deficiency of all three enzymes occurs in individuals with TFP deficiency.
LCHAD and TFP deficiency are disorders of long-chain fatty acid oxidation, which typically present with recurrent episodes of hypoketotic hypoglycemia precipitated by fasting or illness. In addition, the other characteristic manifestations of long-chain fatty acid oxidation defects (FAODs) such as cardiomyopathy, liver dysfunction, or rhabdomyolysis may be present. However, peripheral neuropathy and retinopathy are unique complications of these disorders not seen in other FAODs. The clinical presentation represents a continuous spectrum of severity ranging from severe neonatal-onset to mild late-onset forms. Individuals with LCHAD deficiency usually present with a severe-to-intermediate phenotype, while individuals with TFP deficiency typically present with a severe-to-mild phenotype.
Table 2.
LCHAD/TFP Deficiency: Frequency of Select Features
View in own window
| Feature | ~% of Persons w/Feature 1 | Comment |
|---|
| LCHAD Deficiency | TFP Deficiency |
|---|
Severe neonatal
presentation
| 15% | 39% | Often lethal when assoc w/dilated cardiomyopathy |
Hypoketotic
hypoglycemia
| 78% | 40% | Common in severe neonatal presentation; precipitated by fasting or illness in intermediate phenotype |
Liver
dysfunction
| 80% | 53% | ↑ liver enzymes, cholestasis, or liver failure during metabolic crisis is common in severe & intermediate phenotypes. |
|
Cardiomyopathy
| 65% | 63% | Common in severe neonatal presentation; may be present in untreated intermediate or mild phenotype |
Skeletal
myopathy
| 62% | 72% | Hypotonia, muscle weakness, exercise intolerance, or episodic muscle pain & myoglobinuria; may be isolated finding in mild phenotype |
Peripheral
neuropathy
| 67% | 79% | Long-term complication present in most surviving persons despite early treatment |
|
Retinopathy
| 80% | 12% | Long-term complication that may → vision impairment |
LCHAD = long-chain hydroxyacyl-CoA dehydrogenase; TFP = trifunctional protein
- 1.
Frequencies are approximations from data published prior to the implementation of newborn screening (NBS) [den Boer ME et al 2002, den Boer ME et al 2003, Spiekerkoetter et al 2003, Spiekerkoetter et al 2004]. NBS has enabled earlier diagnosis and improved outcomes.
Neonatal Onset (Severe/Cardiac Phenotype)
The neonatal-onset (severe/cardiac) presentation is more common in individuals with TFP deficiency than in those with LCHAD deficiency. The main manifestations are the following:
Metabolic decompensation. Newborns present within a few days of birth with a Reye-like syndrome presentation: encephalopathy, hypoketotic hypoglycemia, hepatomegaly with elevated transaminases and hepatosteatosis, and lactic acidosis. Hyperammonemia may also be present. The metabolic decompensation is rapidly progressive and requires immediate intervention. The acute metabolic decompensation is often associated with liver dysfunction manifesting as hepatomegaly, elevated liver enzymes, or liver failure.
Neurologic manifestations. Severe neonatal presentation characterized by hypoglycemia and liver dysfunction is usually associated with encephalopathy manifesting as lethargy, poor feeding, seizures, apnea, or coma.
Cardiac manifestations. The severe form is associated with progressive dilated cardiomyopathy manifesting as arrhythmias and cardiac failure. It is associated with very high mortality.
Late Onset (Mild/Neuromyopathic Phenotype)
Individuals with the mild phenotype usually present after infancy with neuromuscular symptoms. The isolated neuromyopathic presentation is typical of mild TFP deficiency and rare in LCHAD deficiency. However, infants with LCHAD deficiency with the intermediate/hepatic phenotype may present later with neuromyopathic symptoms. The common manifestations are the following:
Skeletal myopathy manifests as muscle weakness, exercise intolerance, and hypotonia. Episodic rhabdomyolysis precipitated by prolonged exercise, cold exposure, fasting, or infection is characteristic of this phenotype. These episodes are characterized by diffuse muscle pain, profound weakness, myoglobinuria, and elevations of serum CK (>5x the upper limit of normal), aldolase, aspartate aminotransferase, and alanine transaminase.
Long-Term Complications
Long-term complications in those with the intermediate and late-onset phenotypes include the following:
Peripheral neuropathy is a unique long-term complication of LCHAD/TFP deficiency. Age of onset ranges from infancy to adulthood (median: age ~7 years) [
Grünert et al 2021]. Onset is earlier in individuals with TFP deficiency than in those with LCHAD deficiency. It is progressive and sensorimotor in nature. However, it can be pure sensory or pure motor. Polyneuropathy is described as axonal or axonal with secondary demyelination on electrophysiologic studies. Neuropathy can worsen during metabolic crisis. Early diagnosis and treatment can delay the onset but may not prevent this complication.
Retinopathy, another unique complication, is much more common in individuals with LCHAD deficiency than those with TFP deficiency. It is progressive and correlates with disease severity. Four different stages of retinopathy in LCHAD deficiency have been described [
Tyni et al 1998]:
Stage 1. Normal to diffuse hypopigmentation of the fundus
Stage 2. Pigment clumping in the fovea
Stage 3. Macular pallor and migration of pigmentary changes toward the periphery
Stage 4. Atrophy of the posterior fundus and further peripheral migration of pigmentary changes
Visual impairment is present from stage 3 onward. Hence, retinopathy may be missed if fundal imaging and electroretinogram are not done. Approximately half of individuals with LCHAD deficiency have evidence of retinopathy by age two years. Early diagnosis and treatment can slow the progress but may not prevent this complication [
Fahnehjelm et al 2016].
Pregnancy Complications
Pregnancy complications such as HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome and acute fatty liver of pregnancy are seen in about 15%-25% of pregnancies in women carrying a fetus affected with LCHAD/TFP deficiency [den Boer et al 2002, Spiekerkoetter et al 2003, Karall et al 2015]. The pathophysiology of maternal complications is unclear. One hypothesis is that HELLP syndrome is precipitated by the excessive hydroxyacyl derivatives produced by the affected fetus [Kobayashi et al 2015]. An alternative hypothesis is that maternal heterozygosity for LCHAD/TFP deficiency causes hepatic insufficiency [Blish & Ibdah 2005].
Genotype-Phenotype Correlations
HADHA. Homozygous c.1528G>C variants are associated with LCHAD deficiency. Most individuals with LCHAD deficiency have at least one allele with this variant [Ijlst et al 1996]. In the largest cohort of individuals with LCHAD deficiency, c.1528G>C was present in 84 of 98 alleles. Only one individual was homozygous for another variant [den Boer et al 2002]. However, a few individuals who were compound heterozygous for this variant and another pathogenic variant in HADHA were reported to have TFP deficiency [Grünert et al 2021]. Enzymatic studies were not provided for those individuals. In the absence of homozygosity for this variant, enzyme assay is needed to distinguish between these conditions.
HADHB. In general, individuals with HADHB missense pathogenic variants present with milder phenotypes than those with premature termination or frameshift variants. A missense variant on at least one allele favors the milder phenotype. However, the amino acid p.Arg28 appears critical for TFP function/stability, and variants altering this amino acid lead to the severe presentation when in combination with a severe variant on the other allele [Spiekerkoetter et al 2003].
Although clinical manifestations of HADHA- and HADHB-related TFP deficiency are similar, the distribution of phenotypes differs. Approximately half of individuals with HADHA pathogenic variants present with a severe/lethal phenotype, while 70% of individuals with HADHB variants have a milder phenotype [Spiekerkoetter et al 2004].
Prevalence
The incidence of LCHAD deficiency on NBS data from Australia, Germany, and the US was estimated at 1:250,000; TFP deficiency incidence was estimated at 1:750,000 [Lindner et al 2010].
The carrier frequency of the most common HADHA pathogenic variant in individuals of European ancestry (c.1528G>C) is estimated to be 1:173 in Estonia, 1:217 in Poland, and 1:240 in Finland [Joost et al 2012]. To date, this variant has not been reported in the Japanese or Korean populations [Purevsuren et al 2009].
LCHAD deficiency is especially frequent in the Pomerania region of Poland near the Baltic Sea, partly as a result of a high carrier frequency (1:73) of HADHA variant c.1528G>C in individuals of Kashubian ancestry; the prevalence is estimated at 1:16,900 [Piekutowska-Abramczuk et al 2010, Nedoszytko et al 2017].
Management
A brief outline of treatment recommendations for long-chain fatty acid oxidation defects including long-chain hydroxyacyl-CoA dehydrogenase (LCHAD) / trifunctional protein (TFP) deficiency has been published [Spiekerkoetter et al 2009].
Evaluations Following Initial Diagnosis
To establish the extent of disease and needs in an individual diagnosed with LCHAD/TFP deficiency, the evaluations summarized in Table 4 (if not performed as part of the evaluation that led to the diagnosis) are recommended.
Table 4.
Recommended Evaluations Following Initial Diagnosis in Individuals with LCHAD/TFP Deficiency
View in own window
| System/Concern | Evaluation | Comment |
|---|
Metabolic
decompensation
| Consultation w/metabolic physician/biochemical geneticist & specialist metabolic dietitian | Consider transfer to specialist center w/experience in mgmt of inherited metabolic diseases. |
Blood gas – arterial or venous (e.g., w/i-STAT®), ammonia, lactic acid Glucose, liver transaminases (AST, ALT) Electrolytes w/bicarbonate, BUN, creatinine CK CBC w/differential & addl eval when infection is suspected
| Urgent labs to be obtained if an acute metabolic crisis is suspected |
| Plasma free & total carnitine, plasma acylcarnitine profile, & urine organic acids | To be obtained during period of acute metabolic decompensation, if possible |
|
General
| Referral to clinical geneticist familiar w/LCHAD/TFP deficiency | For implementation of specialized treatment |
|
Cardiology
| Consider cardiology consultation & echocardiography | For eval of cardiomyopathy |
|
Neurology
| Consider neurology consultation | For eval of myopathy & peripheral neuropathy |
|
Ophthalmology
| Consider ophthalmology consultation | For assessment of vision & retinopathy |
|
Development
| Developmental assessment | To incl motor, adaptive, cognitive, & speech/language eval |
Genetic
counseling
| By genetics professionals 1 | To inform affected persons & their families re nature, MOI, & implications of LCHAD/TFP deficiency to facilitate medical & personal decision making |
Family support
& resources
| Assess need for:
| |
ALT = alanine transaminase; AST = aspartate aminotransferase; BUN = blood urea nitrogen; CBC = complete blood count; CK = creatine kinase; LCHAD = long-chain hydroxyacyl-CoA dehydrogenase; MOI = mode of inheritance; TFP = trifunctional protein
- 1.
Medical geneticist, certified genetic counselor, certified advanced genetic nurse
Treatment of Manifestations
Management by multidisciplinary specialists including a metabolic physician / biochemical geneticist, specialist metabolic dietitian, cardiologist, neurologist, ophthalmologist, and developmental pediatrician is recommended.
Table 5.
Treatment of Manifestations in Individuals with LCHAD/TFP Deficiency
View in own window
Manifestation/
Concern | Treatment | Considerations/Other |
|---|
Defect of
long-chain
fatty acid
oxidation
| Avoidance of fasting
Birth-age 3 mos: frequent feeds (every 2-3 hrs) Age 4-12 mos: feeding interval can be ↑ to every 4 hrs if tolerated by 6 mos. From age 6 to 12 mos, daytime feeding interval every 4 hrs; overnight fasting can be gradually ↑ to 6-8 hrs by 12 mos. Age 1-3 yrs: daytime feeding interval 4 hrs; overnight fasting up to 10 hrs may be attempted Age 3+ yrs: overnight fasting up to 12 hrs may be attempted
| ↓ feeding interval by half during periods of illness. After age 1 yr, if preprandial hypoglycemia remains an issue, consider overnight feedings or 1 gm/kg of uncooked cornstarch at bedtime to ensure sufficient glucose supply overnight.
|
| MCT supplementation
| MCT can bypass carnitine shuttle & enter mitochondria directly. As medium-chain fatty acid oxidation is intact, it provides important source of calories & is cornerstone of mgmt in long-chain FAOD. |
Triheptanoin (C7)
Approved by FDA in 2020 for treatment of long-chain FAODs; can be used as an alternative to MCT to provide up to 35% of daily calorie intake. Triheptanoin treatment can ↓ frequency of hospitalizations & rhabdomyolysis 1 & improve cardiomyopathy, hepatomegaly, & hypoglycemia. 2 Adverse effects are mainly gastrointestinal & transient (e.g., abdominal pain, diarrhea).
| Triheptanoin is an odd-chain MCT consisting of 3 7-carbon fatty acids metabolized to acetyl CoA & propionyl CoA. Propionyl CoA provides an anaplerotic effect by replenishing mitochondrial tricarboxylic acid cycle intermediates. Thus, compared to even-chain MCT, triheptanoin provides addl benefits through anaplerosis. 3 |
Secondary
carnitine
deficiency
| L-carnitine: 25-50 mg/kg daily in 3 divided doses | Carnitine supplementation is NOT recommended unless there is carnitine deficiency because of concern for cardiotoxicity of long-chain hydroxyacylcarnitine derivatives. |
Poor weight
gain / FTT
|
| Low threshold for clinical feeding eval &/or radiographic swallowing study if clinical signs or symptoms of dysphagia |
|
DD/ID
| Interventions per developmental pediatrician / neurodevelopment specialist | PT, OT, & speech therapy, as indicated |
Cardiac
dysfunction
| Interventions per cardiologist | Early diagnosis & strict dietary therapy can prevent & even reverse cardiomyopathy. 4 |
Peripheral
neuropathy
| Interventions per neurologist | Early diagnosis & strict dietary therapy may delay onset or slow progression but may not completely prevent this complication. 5 |
|
Retinopathy
| Interventions per ophthalmologist | Early diagnosis & strict dietary therapy may delay onset or slow progression but may not completely prevent this complication. 6 |
CoA = coenzyme A; DD/ID = developmental delay /intellectual disability; FAOD = fatty acid oxidation disorders; FTT = failure to thrive; LCHAD = long-chain hydroxyacyl-CoA dehydrogenase; MCT = medium-chain triglyceride; OT = occupational therapy; PT = physical therapy; TFP = trifunctional protein
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
Table 6.
Emergency Outpatient Treatment in Individuals with LCHAD/TFP Deficiency
View in own window
Manifestation/
Concern | Treatment | Considerations/Other |
|---|
Metabolic
decompensation /
Hypoglycemia 1
| ↓ fasting interval by 1/2 of non-sick-day duration. Encourage intake of sugary drinks (e.g., Gatorade™, juice).
| See Table 6 for recommended maximal fasting intervals at baseline. |
|
Fever
| Administration of antipyretics (acetaminophen, ibuprofen) if temperature rises >38.5° C |
|
Occasional
vomiting
| Antiemetics 2 | Some classes of antiemetics can be used safely on an occasional basis to temporarily improve enteral tolerance of food & beverages at home or during transfer to hospital. |
LCHAD = long-chain hydroxyacyl-CoA dehydrogenase; TFP = trifunctional protein
- 1.
Parents or local hospitals should immediately inform the designated metabolic center if: (1) temperature rises >38.5° C; (2) persistent vomiting/diarrhea or other symptoms of intercurrent illness develop; or (3) new neurologic symptoms occur.
- 2.
Avoid ondansetron and other medications known to prolong QT intervals in individuals with cardiomyopathy and/or long QT intervals.
Acute manifestations (e.g., lethargy, encephalopathy, intractable vomiting, seizures, severe myalgia, red-colored urine) often occur in the setting of intercurrent illness and/or inadequate caloric intake as a result of poor appetite or prolonged fasting, and should be managed with generous caloric and intravenous fluid support in a hospital setting. Suspected infection should be identified and treated immediately.
Table 7.
Acute Inpatient Treatment in Individuals with LCHAD/TFP Deficiency
View in own window
Manifestation/
Concern | Treatment | Considerations/Other |
|---|
|
Hypoglycemia
| IV fluid w/high dextrose content (≥10%) to maintain blood glucose >100 mg/dL. 1 Starting fluid at 1.5x maintenance usually achieves this goal. Glucose infusion rate of 8-12 mg/kg/min is usually needed for young children.
| High-dose glucose is needed to avoid catabolism. If there is hyperglycemia, start insulin infusion rather than reducing glucose infusion rate.
|
Metabolic
acidosis
| For severe metabolic acidosis (pH <7.10), initiate bicarbonate therapy. A common formula for bicarbonate dose: bicarbonate (mEq) = 0.5 x weight (kg) x [desired bicarbonate − measured bicarbonate] Give 1/2 of calculated dose as slow bolus & remaining 1/2 over 24 hrs.
|
|
|
Hyperammonemia
| Hyperammonemia improves w/reversal of catabolism. High-dose glucose infusion w/insulin infusion is helpful in achieving this goal. If severe hyperammonemia & altered mental status persists after above measures, consider extracorporeal toxin removal procedures such as hemodialysis & hemofiltration.
| Although IV sodium benzoate w/sodium phenylacetate have been used in such circumstances, their utility is doubtful. |
|
Rhabdomyolysis
| Start IV fluid containing 10% dextrose & electrolytes as needed at 2x maintenance (in children) to provide adequate hydration & calories & ensure urine output of >3 mL/kg/hr to prevent acute renal failure. 4 For adults, start IV fluid at 400 mL/hour; tailor to maintain urine output of ~200 mL/hr. 5 If there is acute renal failure at presentation, consult nephrologist for hemodialysis.
| Avoid treatment of rhabdomyolysis by glucose-free hypotonic IV fluid such as 0.45 normal saline, as it will promote catabolism & worsen rhabdomyolysis. If hyperglycemia develops due to high dextrose infusion, start insulin infusion.
|
|
Cardiac failure
| Manage cardiac failure due to cardiomyopathy in collaboration w/cardiologist. | Consider triheptanoin for those persons not taking it, as it was reported to be useful in mgmt of acute cardiomyopathy. 6 |
IV = intravenous; LCHAD = long-chain hydroxyacyl-CoA dehydrogenase; TFP = trifunctional protein
- 1.
Monitor blood glucose levels every 1-2 hours initially.
- 2.
Intralipid administration is contraindicated; supplemental calories should be provided in the form of carbohydrates.
- 3.
Note that bicarbonate therapy alone is not sufficient to correct the metabolic acidosis. Correction of metabolic acidosis relies on reversing the catabolic state by providing calorie support from glucose.
- 4.
- 5.
- 6.
Triheptanoin was found to be useful in management of cardiomyopathy in both chronic and acute settings [Vockley et al 2016].
Prevention of Primary Manifestations
Avoidance of fasting and supplementation with medium-chain triglycerides (MCT) or triheptanoin remains the mainstay of treatment. Early diagnosis and strict dietary therapy may prevent or delay the onset or slow the progression of long-term complications [Fletcher et al 2012, Fahnehjelm et al 2016, Immonen et al 2016b, De Biase et al 2017, Fraser et al 2019, Dulz et al 2021, Grünert et al 2021].
Education of parents and caregivers is critical to ensure diligent observation and timely initiation of treatment in the setting of intercurrent illness or other catabolic stressors. Prompt administration of high dextrose-containing intravenous fluids is essential to avoid complications such as hypoglycemia, liver failure, rhabdomyolysis, encephalopathy, and coma.
Written protocols for emergency treatment (see Table 8) should be provided to parents, primary care providers / pediatricians, teachers, and school staff. Emergency letters should summarize key information and principles of emergency treatment for LCHAD/TFP deficiency and contain contact information for the primary treating metabolic center. For any planned travel or vacations, consider contacting a center of expertise near the destination prior to travel dates.
Table 8.
Sample Emergency Management Protocol for Individuals with LCHAD/TFP Deficiency
View in own window
|
Name:
| |
|
Date of birth:
| |
|
Medical record number:
| |
|
Diagnosis
| This person has been diagnosed with long-chain hydroxyacyl CoA dehydrogenase deficiency (LCHAD) / trifunctional protein (TFP) deficiency. LCHAD/TFP deficiency is an inherited disorder of fatty acid metabolism. |
|
Warning signs/symptoms
| Intercurrent infections, poor oral intake, vomiting, or diarrhea can precipitate metabolic decompensation leading to vomiting, lethargy, hypoglycemia, metabolic acidosis, lactic acidosis, and muscle breakdown. Prompt provision of adequate calories (reversal of catabolism) and IV fluids is essential. If not adequately treated, individuals can develop severe hypoglycemia, liver failure, heart failure, muscle breakdown, kidney failure, and permanent neurologic damage. Severe morbidity and even death can occur. |
|
Emergency room management
| Start IV fluid immediately even if not clinically dehydrated with 10% dextrose & appropriate electrolytes at 1.5x maintenance rate. It is imperative to prevent or reverse catabolism immediately. Correct metabolic acidosis by giving sodium bicarbonate if acidosis is severe (pH <7.10 or bicarbonate <10mEq/L). Do not wait for results of laboratory evaluation before starting IV fluids with glucose. Monitor blood glucose levels every 1-2 hours initially and maintain glucose levels at >100 mg/dL.
|
Laboratory evaluation
(and other recommended evaluations) | Urgent labs/procedures:
Blood gas – arterial or venous (e.g., w/i-STAT®), ammonia, lactic acid Glucose, liver transaminases (AST, ALT) Electrolytes with bicarbonate, BUN, creatinine CK CBC with differential & additional evaluation when infection is suspected EKG, echocardiography
Additional labs to be sent if feasible:
|
|
Metabolic center contact
| [Emergency contact phone/pager of the individual's metabolic center should be provided here.]
Stabilization of the affected individual should be the first priority. |
ALT = alanine transaminase; AST = aspartate aminotransferase; BUN = blood urea nitrogen; CBC = complete blood count; CK = creatine kinase; IV = intravenous; LCHAD = long-chain hydroxyacyl-CoA dehydrogenase; TFP = trifunctional protein
Surveillance
There are no current published guidelines for surveillance. In addition to regular evaluations by a metabolic specialist and metabolic dietician, the evaluations in Table 9 are recommended.
Table 9.
Recommended Surveillance for Individuals with LCHAD/TFP Deficiency
View in own window
| Manifestation | Evaluation | Frequency/Comment |
|---|
|
Metabolism
| Nutritional mgmt | At each visit. Frequency of visits is determined by clinical severity. Follow-up interval can be adjusted based on metabolic control. A rough guideline (by age):
<1 yr: weekly to monthly 1-7 yrs: every 1-6 mos >7 yrs: every 6-12 mos
|
| Comprehensive fatty acid profile to assess for essential fatty acid deficiency 1 | Annually |
| Plasma free & total carnitine, acylcarnitine profile, CK, AST, ALT | Recommended frequency (by age):
<1 yr: every 3 mos 1-7 yrs: every 3-6 mos >7 yrs: every 6-12 mos
|
|
Growth
| Measurement of head circumference & growth | At each visit throughout childhood |
|
Development
| Monitoring of developmental milestones |
|
| As needed |
|
Cardiomyopathy
| EKG & echocardiography | Annually or more frequently for severe presentation |
|
Peripheral neuropathy
| Neurology eval | Annually |
| NCV & EMG | As needed |
|
Retinopathy
| Ophthalmology eval | Annually |
| ERG | Every 2-3 yrs |
ALT = alanine transaminase; AST = aspartate aminotransferase; CK = creatine kinase; NCV = nerve conduction velocity test; EMG = electromyography; ERG = electroretinography; LCHAD = long-chain hydroxyacyl-CoA dehydrogenase; TFP = trifunctional protein
- 1.
Affected individuals are at risk of developing essential fatty acid deficiency as a result of a diet restricted in long-chain fat.
Agents/Circumstances to Avoid
Avoid the following:
Fasting, including periods of preparation and recovery from planned surgery or anesthesia
Inadequate caloric provision during stressors, especially when fasting is involved (surgery or procedure requiring fasting/anesthesia)
Inadequate calories following vaccination
Note: Vaccination is safe.
Dehydration (risk for rhabdomyolysis and acute renal failure)
High-fat diet including ketogenic or carbohydrate-restricted diets for the purpose of weight loss, such as Atkins diet
Administration of intravenous intralipids during an acute metabolic crisis
Anesthetics that contain high doses of long-chain fatty acids (e.g., propofol, etomidate) are avoided in long-chain fatty acid oxidation defects. However, a retrospective analysis revealed no adverse events with propofol for short-duration procedures in individuals with LCHAD/TFP deficiency [Martin et al 2014]. A combination of midazolam, thiopental, fentanyl, and remifentanil was used successfully in an individual with LCHAD deficiency [Steinmann et al 2010].
Evaluation of Relatives at Risk
At-risk sibs of any age. Testing of all at-risk sibs of any age is warranted to identify as early as possible those who would benefit from institution of treatment and preventive measures (see Management, Prevention of Primary Manifestations).
If the pathogenic variants in the family are known, molecular genetic testing can be used to clarify the genetic status of at-risk sibs.
If the pathogenic variants in the family are not known, obtain a plasma acylcarnitine profile, plasma free and total carnitine, and urine organic acid profile.
Newborn sibs. For at-risk newborn sibs when prenatal testing was not performed: in parallel with newborn screening (NBS)* either test for the familial HADHA or HADHB pathogenic variants or obtain a plasma acylcarnitine profile, plasma free and total carnitine, and urine organic acid profile.
* The following medical interventions need to begin immediately on receipt of an abnormal NBS result while additional testing is performed to determine whether the abnormal screen represents a true positive NBS result and to establish a definitive diagnosis of LCHAD/TFP deficiency:
Evaluation of the newborn to ascertain clinical status
Education of the caregivers to avoid prolonged fasting and to monitor for decreased oral intake, vomiting, or lethargy
Immediate intervention (to be considered if the newborn is not doing well clinically) possibly including admission to the hospital, fluid resuscitation, infusion of IV dextrose (≥10%), and cardiac evaluation
See Genetic Counseling for issues related to testing of at-risk relatives for genetic counseling purposes.
Pregnancy Management
Labor and postpartum periods are catabolic states and place the mother at higher risk for rhabdomyolysis and myoglobinuria. A successful pregnancy in a female with LCHAD deficiency has been reported [van Eerd et al 2017]. Management included increasing MCT intake in the third trimester and high dextrose infusion in the peripartum period.
Maternal complications such as HELLP syndrome and acute fatty liver of pregnancy are seen in an estimated 15%-25% of pregnancies in women carrying a fetus affected with LCHAD/TFP deficiency [den Boer et al 2002, Spiekerkoetter et al 2003, Karall et al 2015]. Pregnant females who are heterozygous for a HADHA or HADHB pathogenic variant (including suspected carriers) should be monitored for HELLP syndrome and acute fatty liver of pregnancy. Liver function testing should be performed at each prenatal visit during the first two trimesters and more frequently during the third trimester, when the risk for HELLP syndrome and acute fatty liver of pregnancy is the greatest. Management by a team comprising a maternal-fetal medicine specialist and a medical/biochemical geneticist is recommended.
See MotherToBaby for further information on medication use during pregnancy.
Therapies Under Investigation
Cardiac transplantation. Favorable outcome after cardiac transplantation in individuals with TFP deficiency has been reported [Bursle et al 2018]. However, it is expected that with timely diagnosis, strict dietary therapy, and MCT or triheptanoin supplementation, cardiac transplantation may not be required.
Bezafibrate is a hypolipidemic drug and an agonist of peroxisome proliferator-activated receptor (PPAR). It increases expression of several enzymes involved in mitochondrial fatty acid oxidation, including TFP [Djouadi et al 2016]. Bezafibrate was reported to have a favorable outcome in two individuals with TFP deficiency [Suyama et al 2020]. Bezafibrate is not available in the United States.
REN001 (Reneo Pharmaceuticals®) is a selective PPAR-δ agonist that increases transcription of genes involved in mitochondrial fatty acid oxidation. It has received orphan drug designation for LCHAD deficiency from the European Medicines Agency.
Search ClinicalTrials.gov in the US and EU Clinical Trials Register in Europe for access to information on clinical studies for a wide range of diseases and conditions. Note: There may not be clinical trials for this disorder.
Genetic Counseling
Genetic counseling is the process of providing individuals and families with
information on the nature, mode(s) of inheritance, and implications of genetic disorders to help them
make informed medical and personal decisions. The following section deals with genetic
risk assessment and the use of family history and genetic testing to clarify genetic
status for family members; it is not meant to address all personal, cultural, or
ethical issues that may arise or to substitute for consultation with a genetics
professional. —ED.
Mode of Inheritance
Long-chain hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency and trifunctional protein (TFP) deficiency are inherited in an autosomal recessive manner.
Risk to Family Members
Parents of a proband
The parents of an affected child are presumed to be heterozygous for an HADHA or HADHB pathogenic variant.
Molecular genetic testing is recommended for the parents of a proband to confirm that both parents are heterozygous for an HADHA or HADHB pathogenic variant and to allow reliable recurrence risk assessment. If a pathogenic variant is detected in only one parent and parental identity testing has confirmed biological maternity and paternity, the following possibilities should be considered:
Heterozygotes (carriers) are not at risk of developing LCHAD/TFP deficiency. However, pregnant female carriers may be at risk of developing HELLP syndrome and acute fatty liver of pregnancy if the fetus has LCHAD/TFP deficiency (see
Pregnancy Management).
Sibs of a proband
If both parents are known to be heterozygous for an HADHA or HADHB pathogenic variant, each sib of an affected individual has at conception a 25% chance of being affected, a 50% chance of being a carrier, and a 25% chance of inheriting neither of the familial pathogenic variants.
Significant intrafamilial clinical variability may be observed between sibs who inherit the same biallelic
HADHA or
HADHB pathogenic variants. However, this variability is considered to stem primarily from environmental factors such as diet and the severity of infection triggering metabolic decompensation [
Tyni & Pihko 1999,
Bursle et al 2018,
Wei et al 2020].
Heterozygotes (carriers) are not at risk of developing LCHAD/TFP deficiency. However, pregnant female carriers may be at risk of developing HELLP syndrome and acute fatty liver of pregnancy if the fetus has LCHAD/TFP deficiency (see
Pregnancy Management).
Offspring of a proband. The offspring of an individual with LCHAD/TFP deficiency are obligate heterozygotes (carriers) for a pathogenic variant in HADHA or HADHB.
Other family members. Each sib of the proband's parents is at a 50% risk of being a carrier of an HADHA or HADHB pathogenic variant.
Carrier Detection
Molecular genetic carrier testing for at-risk relatives requires prior identification of the HADHA or HADHB pathogenic variants in the family.
Note: Because biochemical analysis is usually normal in carriers, biochemical testing is not reliable for the detection of carriers.
Prenatal Testing and Preimplantation Genetic Testing
Once the HADHA or HADHB pathogenic variants have been identified in an affected family member, prenatal and preimplantation genetic testing are possible.
Differences in perspective may exist among medical professionals and within families regarding the use of prenatal testing. While most centers would consider use of prenatal testing to be a personal decision, discussion of these issues may be helpful.
Molecular Genetics
Information in the Molecular Genetics and OMIM tables may differ from that elsewhere in the GeneReview: tables may contain more recent information. —ED.
Table A.
Long-Chain Hydroxyacyl-CoA Dehydrogenase Deficiency / Trifunctional Protein Deficiency: Genes and Databases
View in own window
Data are compiled from the following standard references: gene from
HGNC;
chromosome locus from
OMIM;
protein from UniProt.
For a description of databases (Locus Specific, HGMD, ClinVar) to which links are provided, click
here.
Table B.
OMIM Entries for Long-Chain Hydroxyacyl-CoA Dehydrogenase Deficiency / Trifunctional Protein Deficiency (View All in OMIM)
View in own window
|
600890 | HYDROXYACYL-CoA DEHYDROGENASE/3-KETOACYL-CoA THIOLASE/ENOYL-CoA HYDRATASE, ALPHA SUBUNIT; HADHA |
|
609015 | MITOCHONDRIAL TRIFUNCTIONAL PROTEIN DEFICIENCY 1; MTPD1 |
|
609016 | LONG-CHAIN 3-HYDROXYACYL-CoA DEHYDROGENASE DEFICIENCY |
|
620300 | MITOCHONDRIAL TRIFUNCTIONAL PROTEIN DEFICIENCY 2; MTPD2 |
Molecular Pathogenesis
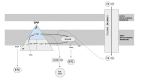
Mitochondrial fatty acid oxidation (beta-oxidation) is the primary pathway of energy production from fatty acids. Fatty acid undergoes repeated cycles of four steps inside mitochondria that result in the shortening of fatty acid by two carbon atoms and production of acetyl-coenzyme A (CoA), reduced nicotinamide adenine dinucleotide (NADH), and reduced flavin adenine dinucleotide (FADH2). Acetyl-CoA can either be utilized to make ketone bodies or oxidized via the tricarboxylic acid cycle for energy generation. High-energy electrons in NADH and FADH2 molecules are transferred to the electron transport chain for ATP generation. The first of these four steps are catalyzed by acyl-CoA dehydrogenase, while the last three are catalyzed by the mitochondrial trifunctional protein (TFP) (see ).
Mitochondrial fatty acid oxidation (beta-oxidation), the primary pathway of energy production from fatty acids Activated long-chain fatty acyl-coenzyme A (CoA) is transported from the cytoplasm across the mitochondrial membrane by the carnitine shuttle. (more...)
Mitochondrial TFP is an octamer composed of four alpha subunits (encoded by HADHA) and four beta subunits (encoded by HADHB). The alpha subunit catalyzes long-chain enoyl-CoA hydratase and long-chain 3-hydroxyacyl-CoA dehydrogenase activities, while the beta subunit catalyzes long-chain 3-ketoacyl-CoA thiolase activity.
In addition, the alpha subunit of TFP participates in cardiolipin remodeling, and TFP physically interacts with mitochondrial respiratory chain complex 1 [Taylor et al 2012, Miklas et al 2019, Wang et al 2019]. Resemblance of TFP deficiency to mitochondrial respiratory chain disorders (e.g., elevated lactic acid, cardiomyopathy, polyneuropathy, retinopathy) may be explained by these functional and physical interactions of TFP with the respiratory chain.
Mechanism of disease causation. Loss of function
Table 10.
Notable HADHA Pathogenic Variants
View in own window
LCHAD = long-chain hydroxyacyl-CoA dehydrogenase
Variants listed in the table have been provided by the authors. GeneReviews staff have not independently verified the classification of variants.
GeneReviews follows the standard naming conventions of the Human Genome Variation Society (varnomen.hgvs.org). See Quick Reference for an explanation of nomenclature.