Introduction
The enormous success of the last two decades in molecular embryology and developmental genetics have afforded fresh views on old problems. For instance, from a developmental point of view there is an alternative way to think about solid cancers that contrast with the single cell focus derived from molecular and cell biology and their emphasis on the cell cycle and the concepts of transformation and oncogene. One can consider cancers as diseases of patterning affecting stem cell lineages or properties. Within this framework, the funnel hypothesis1 suggests that the multiple ways to induce a given type of tumor in the mouse and the many functional mutations identified in a given tumor in humans lead to the activation of a single or few events in the cell that affect its position and identity. A one to one relationship between phenotype and final molecular mechanism with some redundancy is proposed for cancer, much as in embryonic development. Moreover, the ability of pathologists to recognize consistent morphologies in tumors and to give appropriate diagnoses is explained by the action of consistent paradevelopmental programs.1 Concepts like cell competence, specification, niche, developmental history and positional information thus become central to understand, and hopefully treat, cancer. Indeed, under this light, understanding cancer becomes necessary in order to have a complete view of developmental potential.
We can then consider—with caution as we presently lack the perspective that elapsed time affords—what has been learned and achieved on Hedgehog signaling in cancer in recent years. The Hedgehog story is an unfolding one, one that does not yet have a known end, and is only one of several stories,2 but so far it is the most promising one.
This story starts with the discovery of the Hedgehog (Hh) gene in Drosophila melanogaster ˜25 years ago, well before Hh signaling was implicated in cancer. The identification of the Hh gene through its mutation, which causes aberrant embryonic patterning giving the mutant larva a ‘hairy’ aspect, and thus named hedgehog,3 was published in 1980. The cloning of the single Drosophila Hh gene was published by several groups in the early '90s,4-6 with the cloning and identification of Hh genes in vertebrates (Sonic hedgehog (Shh), Indian Hh and Desert Hh in mice and humans) following shortly afterwards.7-10 The definition of the expression patterns of Hh genes in the developing embryo showed that they are often strikingly restricted to cells and areas previously known to have special patterning activities. The exquisite spatio-temporal regulation of Hh genes in the developing embryo and adult tissues, together with the ability of Hh proteins to elicit defined cell fate changes in a concentration-dependent manner highlighted a revolution in molecular embryology, providing mechanistic explanations for critical events in the formation of the animal body plan. This includes for instance, the patterning and growth of limb buds and of the brain and spinal cord.7-13
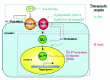
The activity of the Hh pathway involves several steps (Fig. 1).12-14 Secreted Hh glycoproteins act via the transmembrane proteins Patched1 (Ptch1) and Smoothened (Smo). It is thought that Hh binding to Ptch1 inhibits the repression of Smo by Ptch1 so that Smo functions only when Hh is present. Smo in turn transduces the Hh signal intracellularly to activate the zinc-finger Gli transcription factors.13 The Gli proteins are required and sufficient to mediate Hh activity. A number of modulators, positive or negative, some involving negative feedback such Hip1, Gas1 and Ptch1 itself, also regulate Gli activity so that this is tightly controlled in time and space. Because the Hh-Gli pathway is to a large extent linear, activity can be achieved by loss of function of negative factors, such as Ptch1 or the gain of function of positive factors, such as mutations in Smo that may render it insensitive to Ptch1 repression.
The initial link of HH signaling and familial human cancer was published in the mid-1990s15,16 linking mutations in PATCHED1 (PTCH1, an inhibitor of HH signaling; Fig. 1), with the rare Basal Cell Nevus syndrome or Gorlin's syndrome.17 Affected individuals can develop numerous basal cell carcinomas (BCCs) of the skin at an early age as well as medulloblastomas and other tumors, and display additional malformations including odontogenic keratocysts.17 These studies converged on PTCH1 from the study of its function in flies and mammals16 and from skin carcinogenesis.15
The first demonstration of a functional link of HH-GLI signaling with sporadic human cancer was published in 1997, proposing that all human BCCs—the most common type of cancer—derive from the activation of the HH-GLI pathway as all examined BCCs expressed GLI118 (Fig. 1), since its transcription is the best marker of a cell's response to HH signaling.19 In addition to GLI1, GLI2 and PTCH1 (another HH regulated gene20) have also been found to be expressed consistently in sporadic BCCs.18,21-25
GLI1 was discovered as an amplified gene in a human glioma line in 1987.26 This was followed by the discovery of a related gene in vertebrates (GLI327) and independently of a single related gene, Cubitus interruptus (Ci), in Drosophila.28,29 A role for GLI1 in cancer was negated by subsequent findings30,31 and research on GLI1 waned for several years. However, GLI3 was shortly afterwards identified as the gene mutated in Greig's Cephalopolydactily syndrome32-34 and later implicated in Pallister-Hall syndrome.35,36 Patients affected with these diseases display a wide range of phenotypes, including additional digits, skull defects, imperforate anus and hypothalamic growths (hamartomas). The misregulation of post-transcriptionally cleaved C-terminally-deleted forms of GLI3 acting as dominant repressors,37-41 as first shown for Ci,42 has been implicated in these syndromic phenotypes with debated genotype-phenotype correlations.43,44,228 Aberrant GLI function has also been implicated in other syndromic phenotypes, including VACTERL,45,46 a syndrome that includes vertebral anomalies, anal atresia, cardiac defects, tracheoesophageal fistula, and renal and limb defects.
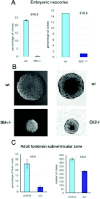
Three Gli genes were then cloned from different species, including mice,47-49 chicks,50 frogs,19,51 and zebrafish.52,53 They were originally shown to affect neural patterning by mimicking Shh.19,54 In addition, microinjection of human or frog Gli1 lead to sporadic epidermal and CNS tumors or hyperplasias (Fig. 2).18,55 Interpretation of GLI1-induced epidermal hyperplasias as tumors related to human BCCs awaited the papers on the involvement of loss of PTCH1 in Gorlin's syndrome15,16 in which patients develop numerous BCCs.17
Following the finding of familial mutations in PTCH1 in Gorlin's syndrome patients15,16 and simultaneous with the involvement of HH-GLI signaling in sporadic cancer,18 mutations in the HH-GLI pathway components PTCH1 and SMOH (Fig. 1) were sought and found in a small fraction of sporadic skin, brain and other tumors,21,56-67 some of which have been proven to have functional relevance.62 There are also reported mutations in Suppressor of fused68 (SUFUH; but see ref. 69), a negative modulator of GLI function.70-72 Mutations in the GLI genes have not been found in tumors, with the exception of beta-actin-GLI1 fusions in a subset of pericytomas.73 These findings provide possible bases for the activation of the HH pathway in a fraction of tumors although many others might derive from epigenetic events.
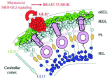
Various animal models demonstrating the sufficiency of an active Hh-Gli pathway to induce tumor formation (Table 1, Fig. 2) began to be published in 1997 for skin (Shh;74 Gli1;18,75,76 Smo;62,77 Ptch1;78 Gli277,79-81), brain (Ptch1;82,83 Gli1,55 Shh+c-Myc;221 Shh+IGF2;222 Shh+Akt222) and muscle (Ptch183). At the same time, a rational context for the various human tumors now associated with HH-GLI signaling began to be uncovered with the realization that elements of this pathway are expressed in the corresponding normal tissues and that HH-GLI signaling controls growth, patterning and/or homeostasis of developing and adult organs such as the skin18,74,84,85 and the cerebellum86-88 (Fig. 3). Indeed, the control of cell proliferation in the late embryonic and perinatal dorsal brain by SHH-GLI signaling may account for its involvement in many types of CNS tumors.55
Table 1
Human cancers dependent on sustained HH-GLI signaling.
Shh-Gli signaling is not only required for the normal expansion of many types of progenitors but it is also required for the control of neural stem cell behavior in the perinatal and adult rodent brain, including the embryonic neocortex89 (Fig. 4), the perinatal and adult hippocampus90,91 and the subventricular zone of the lateral ventricle of the forebrain91,92 (Fig. 4). Shh also regulates the proliferation of embryonic spinal cord precursors.93 Similarly, there is evidence that Hh signaling regulates precursor/stem cell lineages and morphogenesis in other tissues and organs, such as the intestine94-96 and perhaps the skin.81,97 Together, these findings have raised the possibility that tumors derive from HH-sensitive stem cell lineages, possibly acting at the level of stem cells in some cases or at the level of derived early precursors.
Generally, the best evidence that stem cells exist in tumors come from the transfer of leukemia from a sick mouse to a healthy recipient by the implantation of a few rare cancer stem cells98-100 and the impressive findings that teratocarcinoma cells can contribute to the development of an entire fertile mouse when transplanted into the inner cell mass of a recipient embryo.101-103 Here, cancer would appear to be induced by genetic/epigenetic changes that are reversed in the appropriate context or niche. These original experiments demonstrate the stemness of teratocarcinoma cancer cells and their totipotency.
For adult solid tumors, evidence in favor of the existence of cancer stem-like cells derives from the finding that cells with self-renewal properties have been reported in tumor cells from brain and breast.104-110 Since a small number of glioma cells show clonogenic properties that are regulated by HH signaling (P. Sanchez, V. Clément and ARA, pers. com.) stem cell lineages (stem cells and/or their early derived more restricted progeny) may thus be the target of carcinogens, mutations and epigenetic changes that lead to the initiation of tumorigenesis in the brain and other HH-responsive organs and tissues, such as the epithelial compartment of the prostate, lung or gastrointestinal tract.
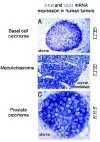
The first demonstration that human sporadic cancers require sustained HH-GLI signaling for continued growth derived from the ability to inhibit the proliferation of medulloblastoma cells by blocking HH signaling.55 Since then, a rapidly increasing number of sporadic human tumors has been found to express GLI1 and sometimes SHH (Fig. 5), and to be dependent on sustained HH-GLI pathway function (Table 2). These include basal cell carcinomas;18,111,224 medulloblastomas,55,112 gliomas55 (P. Sanchez, V. Clément and ARA, pers. com), pancreatic tumors,113,114 small cell lung cancer,115 stomach tumors116 and prostate tumors.117-120 Additional studies show that a variety of (benign and malignant) tumor cells express HH-GLI pathway components, that tumors are correlated with mutation in genes that affect the pathway and/or that tumor cell lines are sensitive to inhibition of HH signaling. These include breast tumors,121 sarcomas,122 ameloblastomas,123 a subset of pericytomas,73 astrocytomas,55,124 oral squamous cell carcinomas,125 bone exostoses,126 neurofibromas,127 bladder cancer,128 colorectal tumors129-132 and plasmacytomas220 among others. The number of tumors and benign tumor-like growths, such as odontogenic keratocycts,133 in which HH-GLI signaling may play a critical role is presently growing at an impressive rate. For example, tumors of liver, colon, rectum, lung and stomach are reported to have low levels of Hedgehog-interacting protein134 (HIP1), a HH signaling antagonist.135
Table 2
Vertebrate animal models of HH-GLI dependent cancers.
The rationale for the initial testing for the requirement of sustained HH-GLI function for tumor maintenance first derived from the timing of appearance of somatic GLI1-induced tumors in tadpoles18,55 and from the finding that the activity of mutations in SMOH and PTCH1 is inhibited by cyclopamine,136 although the latter cannot distinguish between an involvement in tumor initiation or tumor maintenance. The appearance of GLI1-induced tadpole tumors (or hyperplasias) occurred well after all the injected material was degraded,55 suggesting the presence of ‘memory’. As the endogenous Gli1 gene was found to be expressed in the induced tumors, it was hypothesized that the activation of the endogenous pathway leading to Gli1 expression was involved in tumor development and could account for the delay in tumor appearance. To test this idea, a morpholino-modified antisense oligonucleotide specific for the endogenous Gli1 mRNA (selectively inhibiting its translation) was coinjected with synthetic human GLI1 RNA. Coinjected embryos developed normally without tumors, demonstrating the requirement of the endogenous Gli1 protein for tumor development.55 This result prompted us to originally test the requirement of sustained signaling for tumor maintenance in humans, even if Gli1 was reported to be redundant in mice.137,138
The requirement of sustained signaling for tumor maintenance and growth has been also recently demonstrated in mice. Conditional expression of Gli2 in basal skin cells leads to the formation of BCCs, which regress when the expression is turned off after tumor formation.81 This result is in line with previous data on the dependence of mouse tumors on the maintained expression of the inducing oncogene.139-141
Inhibition of HH-GLI function in human tumor cells was first achieved with cyclopamine,55 is a plant alkaloid with an overall resemblance to cholesterol that is produced by Veratrum californicum, a corn lily.142-145 It was named cyclopamine because it is the active compound, along with jervine, responsible for the production of cyclopic embryos and newborns after ingestion of V. californicum by pregnant range and farm animals.144,146,147 Cyclopamine-induced cyclopia phenocopies the loss of Shh in mice148 and humans149,150 and it selectively inhibits the HH-GLI pathway,152 binding and blocking the function of SMOH (Fig. 1).153
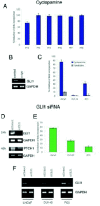
Inhibition of HH-GLI signaling by noncyclopamine small molecule agents154,155 that, like cyclopamine, target SMOH has been achieved in mouse basal cell carcinoma punch explants and cell lines.111,155 Antibodies against SHH156 and immunization with HIP1 have also been used.116,117,157 At the other end of the signaling pathway, inhibition has been achieved by targeting Gli1 with antisense oligonucleotides in skin and CNS tumors in tadpoles55 and with siRNAs in human prostate cancer117 (Fig. 6) and in brain tumor cells (P. Sanchez, V. Clément and ARA, pers. com). Targeting GLI1 offers that best therapeutic outlook as this would inhibit activation of the pathway by any event acting at any level. For instance, alterations in SUFUH have been observed in different tumors68,119 and tumors arising from such events would be insensitive to inhibitors acting on SMOH, as SUFUH acts downstream of SMOH. Indeed, tumor cells insensitive to cyclopamine but dependent on GLI1, as assessed by RNA interference (RNAi), have been found.117
Inhibition of cancer growth through interference with HH-GLI signaling has been shown for established cell lines and for primary cultures from the operating room55,112,113,115-118 (Table 2) as well as for mouse grafts.112,113,115,116,118 It is important to note, however, that while orthografts are valuable, the predictive value of subcutaneous xenografts and allografts is questionable at best and equal or inferior to primary cell cultures.158,159
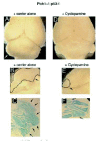
Genetic mouse models of endogenous tumors, however, provide excellent tools for preclinical assessments in a mammal. In this sense, the efficient development of medulloblastomas in all Ptch1+/-;p53-/- mice160 provides a valuable model with a genetically engineered endogenous tumor. Using this model it has been recently shown that systemic interference of HH signaling in Ptch1+/-;p53-/- adult mice leads to tumor inhibition and regression using a synthetic small molecule antagonist161 of SMOH function or using the natural alkaloid cyclopamine162 (Fig. 7). Similarly, oral cyclopamine treatment was preventive for uv-induced BCC development in Ptch1+/- mice.224 Together, these findings set the stage for the initiation of clinical trials on human patients suffering from incurable terminal cancers such as metastatic prostatic and pancreatic tumors or glioblastoma multiforme.
Inhibition of HH-GLI function appears to be effective for all tumor grades of all ages and subgroups of a given type that is HH-GLI signaling dependent. For example, primary culture of in situ prostate tumors of different grades and cells lines derived from distant metastatic lesions117 (Figs. 5, 6), as well as primary culture of fresh prostatic metastatic lesions from postmortem specimens,118 are sensitive to cyclopamine. These and similar findings in other tumor types is amazing and suggest that the HH-GLI pathway is an essential basis on which a tumor is built and that no tumor cell can exist without sustained signaling, be it autocrine or paracrine, leading to positive GLI function (Fig. 8). HH-GLI signaling may thus be a critical sensor of the ‘fitness’ of a cell, with tumor cells requiring a high fitness.
Metastatic lesions may derive from the acquisition of cell-autonomous pathway activation allowing efficient activity independent of the original niche117 and/or from increased overall signaling levels (Fig. 8).118 High levels of GLI1 function lead to high metastatic behavior in xenografts.118 Intrinsic/elevated signaling may then affect genes involved in epithelial-mesenchymal transitions, such as C-Myc, N-Myc163 and Snail,164 genes which are responsive to Hh-Gli activity118,165-167 to start the metastatic voyage and departure from the original tumorigenic niche. c-Myc also cooperates with Shh signaling in tumorigenesis.221 The context of the site of origin of the tumor might also determine the site of metastatic growth, perhaps not just in a random selective manner but also in a directed way, possibly recapitulating aspects of normal cell migration, such as that of the neural crest during embryogenesis.
Unexpectedly for its protocols and expediency, two publications report beneficial effects of cyclopamine on BCCs and psoriasis in human patients.168,169 If confirmed and extended, these results are in perfect agreement with the expected outcome of interference with HH signaling as a novel therapeutic approach in humans. Toxicology and side effect issues need to be addressed before general and systemic treatments can be started but cyclopamine could indeed be a perfect leading compound.
Systemic cyclopamine treatment could cause a number of side effects that might include problems with the gastric mucosae, hair growth or renewal and production of new neurons. However, these are not worse than the side effects of present-day chemotherapy and local, topical cyclopamine treatment did not affect hair follicles adversely.168 Moreover, a minimal treatment period required to induce growth arrest and death of tumor cells may result in transient secondary effects that can be compensated following cessation of the treatment: mice treated for several weeks with cyclopamine162,224 or a different SMOH antagonist161 appear normal.
In principle, however, the best therapies could derive from inhibition of GLI function (Fig. 1), which as it is the last element of the pathway would appear to be the best target. Moreover, GLI proteins are sufficient for tumor induction in animal models18,55,75,79 and might respond to additional tumorigenic inputs other than HH.170 Given that the GLI code13,171,172 is critical for cell fate and cancer, and that it is modulated by several kinases (Fused, PKA, GSK3β, CK1 and GRK2)173-183 and many cofactors, inhibitors of GLI function may target the GLI proteins themselves or such modifying proteins (Fig. 1). These include DYRK1,184 intraflagellar transport proteins,185 ZIC proteins,186,187 REN,188 DZIP1 (Iguana),189,190 RAB23,191 FKBP8192 and SUFUH70-72,193,194 and BEGH.223 Approaches with antagonist (for positive factors) or agonist (for negative regulators) small molecules as discussed above, peptides mimicking dominant-repressive C-terminus deleted GLI proteins37,38 and RNA interference117 (Fig. 7) will very likely lead to the production of efficient, specific and potent inhibitors for patient therapy. Additional beneficial strategies could derive from finding the key targets regulated by HH-GLI signaling the inhibition of which may partially recapitulate loss of activating GLI function. Moreover, large-scale gene profiling, microchip-based diagnoses195 and individual treatment regiments could also lead to more focused and thus better outcomes.
Given the results summarized here, it is difficult to temper enthusiasm for the development of a rational wide-spectrum tumor therapy derived from research on molecular embryology, plant-induced teratology, cancer biology and developmental genetics. Such a therapy would target cancers from brain, lung, pancreas, prostate, muscle, stomach, skin and other organs and of any grade, including most importantly metastatic cancer.
This story is still ongoing and a conclusion has not been reached. At present, pressing questions include the following. How many different types of human tumors depend on HH-GLI signaling? Do tumors derive from stem cells and/or from de-differentiating cells that acquire stem cell properties? What are the paradevelopmental programs that dictate tumor initiation, growth and metastasis? Do HH proteins act as mitogens and/or morphogens in the control of stem cell behavior? Do they contribute to the definition of a normal and tumorigenic stem cell niche? Is there a specific GLI code for each tumor type or a general one that is modified in a context-dependent manner by local cofactors? Is there a correlation of levels of GLI expression/function with tumor grade or character? What are the targets that execute the programs instructed by GLI proteins? Can specific and effective inhibitors of HH-GLI function be produced for patient treatment? Will these have limited and tolerable side effects? Will HH-GLI inhibitors also affect and kill cancer stem cells, thought to be responsible for recurrence? Can cancer result from repeated injury and the eventual perversion of the behavior of stem cells recruited for repair? Can one develop not only anti-cancer but also preventive therapies?
Other questions for which we begin to have some insights include: Do other oncogenic inputs affect or regulate HH-GLI function or vice versa? What are the mechanisms that guide cancer-causing events towards regeneration in some instances? We know that there are several intercellular communication systems that have been implicated in cancer, including FGF, WNT, NOTCH and TGFβ signaling.2 How these interact with HH-GLI function is not yet clear although we know, for instance, that Gli proteins regulate batteries of Wnt genes,196 that FGF signaling can regulate Gli2 and Gli3170 and that Gli2 can be responsive to Notch signaling.197,198 And as far as cancer and regeneration is concerned, insights derive from a variety of sources. For example, on the one hand, treatment of a newt with highly tumorigenic compounds after lens extirpation leads to normal regeneration of the lens from the dorsal iris, but tumor formation from the ventral, nonregenerative ventral iris.199 And on the other hand, some mammalian tumors can be induced to differentiate. For instance, Shh- and Gli2-induced BCCs can differentiate into hair follicle components if placed adjacent to a normal environment,74,81 and teratocarcinomas participate in the formation of a normal mouse when transplanted into a blastocyst.101-103 The developmental history, competence and position of a cell could thus turn tumorigenic into (re)generative information (and vice versa!).
More specifically, these issues on signaling, pattern formation and cancer have lead to renewed interest in homeobox genes. The demonstration of the control of Xhox3 (Evx1) expression by Gli2 and Gli3,170 the regulation of HoxD genes by Gli3,200,201 a physical and functional interaction between HoxD12 and Gli3202 and the genetic interactions between Proboscipedia (HoxA2/B2) and Ci,203 the Gli homologue in Drosophila, suggests the possibility that cell number may be organized to lead to pattern through an interaction between Gli and homeobox proteins. The latter being often regulated by extracellular inputs and best known as organizers of body pattern.204,205 This could explain several unresolved observations, for example, the role of HoxB1 in the control of Shh-mediated D-V pattern in rhombomere 1,206 the ability of HoxB4 to induce hematopoietic stem cell proliferation,207,208 and the involvement of Msx1 in Shh signaling during tooth development.209 The roles of HH-GLI signaling in cancer and stem cell/precursor proliferation (see above), together with the documented participation of homeobox proteins in cancer,210,211 raise the possibilities that these factors regulate size and shape in embryos and homeostasis and regeneration in adults, and that their regulation underlies a unified principle in cancer and development. This hypothesis could also explain evolutionary constrains in body plan and cancer selection.212 Patterning targets/cofactors, such as HOX proteins, in addition to the GLI proteins and their modulators, could thus also be interesting targets of anti-cancer, preventive and pro-regenerative therapies.
In contrast with the inappropriate activation and/or maintenance HH-GLI signaling in cancer, its ability to regulate neurogenesis and brain stem cell behavior,89-92,186,213 raises new and exciting prospects to increase cell number by acting positively on this signaling pathway. This could be critical to increase the pool of cells that is undergoing degeneration, and thus reverse cell loss as in Parkinson's disease,214 or to provide exogenous stem cells to increase repair. Indeed, the ability of SHH to act as a motor neuron survival factor215 and of SHH and GLI1 to act as protective factors for dopaminergic neurons216,217 suggest novel therapies, which may also include the manipulation of homeobox proteins.226,227 Given the adult expression of components of the HH-GLI signaling pathway in the adult brain it is not inconceivable that its deregulation could be involved in additional mental diseases and disorders. Clearly, much awaits to be discovered although the data gathered up to this point already strongly suggest that manipulation of the SHH-GLI1 pathway will lead to cancer treatment, rationally reducing cell number, and to the treatment of degenerative diseases, rationally increasing cell number. Current results are very encouraging and enthusiasm is high. With caution, the goal of developing rational therapies based on knowledge derived from research on flies, lilies, tadpoles, mice, stem cells and human disease is within reach.
Acknowledgements
I am very grateful to Nadia Dahmane, Barbara Stecca, Pilar Sánchez, Verónica Palma, José Mullor, Virginie Clement, Christophe Mas and Ivan Radovanovic for discussion and/or comments on the manuscript, and to all present and previous lab members for sustained intellectual excitement. Work from the author's laboratory was supported by grants from the NIH, Pew Scholars Program, Concern Foundation, Jeantet Foundation, March of Dimes and a Hirschl Award. The reader is encouraged to perform PubMed searches to complement the cited literature and establish time lines as not all references could be included.
References
- 1.
- Ruiz i Altaba A, Stecca B, Sanchez P. Hedgehog-Gli signaling in brain tumors: Stem cells and paradevelopmental programs in cancer. Cancer Lett. 2004;204:145–57. [PubMed: 15013214]
- 2.
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99. [PubMed: 15286780]
- 3.
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. [PubMed: 6776413]
- 4.
- Mohler J, Vani K. Molecular organization and embryonic expression of the hedgehog gene involved in cell-cell communication in segmental patterning of Drosophila. Development. 1992;115:957–71. [PubMed: 1280560]
- 5.
- Lee JJ, von Kessler DP, Parks S. et al. Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell. 1992;71:33–50. [PubMed: 1394430]
- 6.
- Tabata T, Eaton S, Kornberg TB. The Drosophila hedgehog gene is expressed specifically in posterior compartment cells and is a target of engrailed regulation. Genes Dev. 1992;6:2635–45. [PubMed: 1340474]
- 7.
- Riddle RD, Johnson RL, Laufer E. et al. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–16. [PubMed: 8269518]
- 8.
- Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–44. [PubMed: 8269519]
- 9.
- Echelard Y, Epstein DJ, St-Jacques B. et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–30. [PubMed: 7916661]
- 10.
- Roelink H, Augsburger A, Heemskerk J. et al. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell. 1994;76:761–75. [PubMed: 8124714]
- 11.
- Jessell TM. Neuronal specification in the spinal cord: Inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. [PubMed: 11262869]
- 12.
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001;15:3059–3087. [PubMed: 11731473]
- 13.
- Ruiz i Altaba A, Nguyen V, Palma V. The emergent design of the neural tube: Prepattern, SHH morphogen and GLI code. Curr Opin Genet Dev. 2003;13:513–521. [PubMed: 14550418]
- 14.
- Lum L, Beachy PA. The Hedgehog response network: Sensors, switches, and routers. Science. 2004;304:1755–9. [PubMed: 15205520]
- 15.
- Hahn H, Wicking C, Zaphiropoulous PG. et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. [PubMed: 8681379]
- 16.
- Johnson RL, Rothman AL, Xie J. et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. [PubMed: 8658145]
- 17.
- Gorlin RJ. Nevoid basal cell carcinoma (Gorlin) syndrome. Genet Med. 2004;6:530–9. [PubMed: 15545751]
- 18.
- Dahmane N, Lee J, Robins P. et al. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389:876–881. [PubMed: 9349822]
- 19.
- Lee J, Platt KA, Censullo P. et al. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997;124:2537–2352. [PubMed: 9216996]
- 20.
- Goodrich LV, Johnson RL, Milenkovic L. et al. Conservation of the hedgehog/patched signaling pathway from flies to mice: Induction of a mouse patched gene by Hedgehog. Genes Dev. 1996;10:301–12. [PubMed: 8595881]
- 21.
- Gailani MR, Stahle-Backdahl M, Leffell DJ. et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78–81. [PubMed: 8782823]
- 22.
- Unden AB, Zaphiropoulos PG, Bruce K. et al. Human patched (PTCH) mRNA is overexpressed consistently in tumor cells of both familial and sporadic basal cell carcinoma. Cancer Res. 1997;57:2336–40. [PubMed: 9192803]
- 23.
- Green J, Leigh IM, Poulsom R. et al. Basal cell carcinoma development is associated with induction of the expression of the transcription factor Gli-1. Br J Dermatol. 1998;139:911–5. [PubMed: 9892966]
- 24.
- Zedan W, Robinson PA, Markham AF. et al. Expression of the Sonic Hedgehog receptor “PATCHED” in basal cell carcinomas and odontogenic keratocysts. J Pathol. 2001;194:473–7. [PubMed: 11523056]
- 25.
- Tojo M, Kiyosawa H, Iwatsuki K. et al. Expression of the GLI2 oncogene and its isoforms in human basal cell carcinoma. Br J Dermatol. 2003;148:892–7. [PubMed: 12786818]
- 26.
- Kinzler KW, Bigner SH, Bigner DD. et al. Identification of an amplified, highly expressed gene in a human glioma. Science. 1987;236:70–73. [PubMed: 3563490]
- 27.
- Ruppert JM, Vogelstein B, Arheden K. et al. GLI3 encodes a 190-kilodalton protein with multiple regions of GLI similarity. Mol Cell Biol. 1990;10:5408–15. [PMC free article: PMC361243] [PubMed: 2118997]
- 28.
- Orenic TV, Slusarski DC, Kroll KL. et al. Cloning and characterization of the segment polarity gene cubitus interruptus Dominant of Drosophila. Genes Dev. 1990;4:1053–67. [PubMed: 2166702]
- 29.
- Eaton S, Kornberg TB. Repression of ci-D in posterior compartments of Drosophila by engrailed. Genes Dev. 1990;4:1068–77. [PubMed: 2384212]
- 30.
- Salgaller M, Pearl D, Stephens R. In situ hybridization with single-stranded RNA probes to demonstrate infrequently elevated gli mRNA and no increased ras mRNA levels in meningiomas and astrocytomas. Cancer Lett. 1991;57:243–53. [PubMed: 1827754]
- 31.
- Xiao H, Goldthwait DA, Mapstone T. A search for gli expression in tumors of the central nervous system. Pediatr Neurosurg. 1994;20:178–82. [PubMed: 8204491]
- 32.
- Vortkamp A, Gessler M, Grzeschik KH. GLI3 zinc-finger gene interrupted by translocations in Greig syndrome families. Nature. 1991;352:539–40. [PubMed: 1650914]
- 33.
- Schimmang T, Lemaistre M, Vortkamp A. et al. Expression of the zinc finger gene Gli3 is affected in the morphogenetic mouse mutant extra-toes (Xt). Development. 1992;116:799–804. [PubMed: 1289066]
- 34.
- Hui CC, Joyner AL. A mouse model of greig cephalopolysyndactyly syndrome: The extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat Genet. 1993;3:241–6. [PubMed: 8387379]
- 35.
- Kang S, Graham Jr JM, Olney AH. et al. GLI3 frameshift mutations cause autosomal dominant Pallister-Hall syndrome. Nat Genet. 1997;15:266–8. [PubMed: 9054938]
- 36.
- Radhakrishna U, Wild A, Grzeschik KH. et al. Mutation in GLI3 in postaxial polydactyly type A. Nat Genet. 1997;17:269–71. [PubMed: 9354785]
- 37.
- Ruiz i Altaba A. Gli proteins encode context-dependent positive and negative functions: Implications for development and disease. Development. 1999;126:3205–3216. [PubMed: 10375510]
- 38.
- Shin SH, Kogerman P, Lindstrom E. et al. GLI3 mutations in human disorders mimic Drosophila cubitus interruptus protein functions and localization. Proc Natl Acad Sci USA. 1999;96:2880–4. [PMC free article: PMC15863] [PubMed: 10077605]
- 39.
- Sasaki H, Hui C, Nakafuku M. et al. A binding site for Gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development. 1997;124:1313–22. [PubMed: 9118802]
- 40.
- Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–34. [PubMed: 10693759]
- 41.
- Aza-Blanc P, Lin HY, Ruiz i Altaba A. et al. Expression of the vertebrate Gli proteins in Drosophila reveals a distribution of activator and repressor activities. Development. 2000;127:4293–4301. [PubMed: 10976059]
- 42.
- Aza-Blanc P, Ramirez-Weber FA, Laget MP. et al. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89:1043–53. [PubMed: 9215627]
- 43.
- Radhakrishna U, Bornholdt D, Scott HS. et al. The phenotypic spectrum of GLI3 morphopathies includes autosomal dominant preaxial polydactyly type-IV and postaxial polydactyly type-A/B; No phenotype prediction from the position of GLI3 mutations. Am J Hum Genet. 1999;65:645–55. [PMC free article: PMC1377970] [PubMed: 10441570]
- 44.
- Kalff-Suske M, Wild A, Topp J. et al. Point mutations throughout the GLI3 gene cause Greig cephalopolysyndactyly syndrome. Hum Mol Genet. 1999;8:1769–77. [PubMed: 10441342]
- 45.
- Killoran CE, Abbott M, McKusick VA. et al. Overlap of PIV syndrome, VACTERL and Pallister-Hall syndrome: Clinical and molecular analysis. Clin Genet. 2000;58:28–30. [PubMed: 10945658]
- 46.
- Kim J, Kim P, Hui CC. The VACTERL association: Lessons from the Sonic hedgehog pathway. Clin Genet. 2001;59:306–15. [PubMed: 11359461]
- 47.
- Walterhouse D, Ahmed M, Slusarski D. et al. Gli, a zinc finger transcription factor and oncogene, is expressed during normal mouse development. Dev Dyn. 1993;196:91–102. [PubMed: 8364225]
- 48.
- Hui CC, Slusarski D, Platt KA. et al. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev Biol. 1994;162:402–13. [PubMed: 8150204]
- 49.
- Hughes DC, Allen J, Morley G. et al. Cloning and sequencing of the mouse Gli2 gene: Localization to the Dominant hemimelia critical region. Genomics. 1997;39:205–15. [PubMed: 9027508]
- 50.
- Marigo V, Johnson RL, Vortkamp A. et al. Sonic hedgehog differentially regulates expression of GLI and GLI3 during limb development. Dev Biol. 1996;180:273–83. [PubMed: 8948590]
- 51.
- Marine JC, Bellefroid EJ, Pendeville H. et al. A role for Xenopus Gli-type zinc finger proteins in the early embryonic patterning of mesoderm and neuroectoderm. Mech Dev. 1997;63:211–25. [PubMed: 9203143]
- 52.
- Karlstrom RO, Talbot WS, Schier AF. Comparative synteny cloning of zebrafish you-too: Mutations in the Hedgehog target gli2 affect ventral forebrain patterning. Genes Dev. 1999;13:388–93. [PMC free article: PMC316467] [PubMed: 10049354]
- 53.
- Karlstrom RO, Tyurina OV, Kawakami A. et al. Genetic analysis of zebrafish gli1 and gli2 reveals divergent requirements for gli genes in vertebrate development. Development. 2003;130:1549–64. [PubMed: 12620981]
- 54.
- Hynes M, Stone DM, Dowd M. et al. Control of cell pattern in the neural tube by the zinc finger transcription factor and oncogene Gli-1. Neuron. 1997;19:15–26. [PubMed: 9247260]
- 55.
- Dahmane N, Sanchez P, Gitton Y. et al. The Sonic Hedgehog-Gli pathway regulates dorsal brain growth and tumorigenesis. Development. 2001;128:5201–5212. [PubMed: 11748155]
- 56.
- Unden AB, Holmberg E, Lundh-Rozell B. et al. Mutations in the human homologue of Drosophila patched (PTCH) in basal cell carcinomas and the Gorlin syndrome: Different in vivo mechanisms of PTCH inactivation. Cancer Res. 1996;56:4562–5. [PubMed: 8840960]
- 57.
- Raffel C, Jenkins RB, Frederick L. et al. Sporadic medulloblastomas contain PTCH mutations. Cancer Res. 1997;57:842–5. [PubMed: 9041183]
- 58.
- Pietsch T, Waha A, Koch A. et al. Medulloblastomas of the desmoplastic variant carry mutations of the human homologue of Drosophila patched. Cancer Res. 1997;57:2085–8. [PubMed: 9187099]
- 59.
- Vorechovsky I, Tingby O, Hartman M. et al. Toftgard R Somatic mutations in the human homologue of Drosophila patched in primitive neuroectodermal tumours. Oncogene. 1997;15:361–6. [PubMed: 9233770]
- 60.
- Wolter M, Reifenberger J, Sommer C. et al. Mutations in the human homologue of the Drosophila segment polarity gene patched (PTCH) in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 1997;57:2581–5. [PubMed: 9205058]
- 61.
- Xie J, Johnson RL, Zhang X. et al. Mutations of the PATCHED gene in several types of sporadic extracutaneous tumors. Cancer Res. 1997;57:2369–72. [PubMed: 9192811]
- 62.
- Xie J, Murone M, Luoh SM. et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–2. [PubMed: 9422511]
- 63.
- Maesawa C, Tamura G, Iwaya T. et al. Mutations in the human homologue of the Drosophila patched gene in esophageal squamous cell carcinoma. Genes Chromosomes Cancer. 1998;21:276–9. [PubMed: 9523206]
- 64.
- Reifenberger J, Wolter M, Weber RG. et al. Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 58:1798–803. [PubMed: 9581815]
- 65.
- Gemmill RM, West JD, Boldog F. et al. The hereditary renal cell carcinoma 3;8 translocation fuses FHIT to a patched-related gene, TRC8. Proc Natl Acad Sci USA. 1998;95:9572–7. [PMC free article: PMC21380] [PubMed: 9689122]
- 66.
- McGarvey TW, Maruta Y, Tomaszewski JE. et al. PTCH gene mutations in invasive transitional cell carcinoma of the bladder. Oncogene. 1998;17:1167–72. [PubMed: 9764827]
- 67.
- Aboulkassim TO, LaRue H, Lemieux P. et al. Alteration of the PATCHED locus in superficial bladder cancer. Oncogene. 2003;22:2967–71. [PubMed: 12771948]
- 68.
- Taylor MD, Liu L, Raffel C. et al. Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31:306–10. [PubMed: 12068298]
- 69.
- Koch A, Waha A, Hartmann W. et al. No evidence for mutations or altered expression of the Suppressor of Fused gene (SUFU) in primitive neuroectodermal tumours. Neuropathol Appl Neurobiol. 2004;30:532–9. [PubMed: 15488029]
- 70.
- Ding Q, Fukami S, Meng X. et al. Mouse suppressor of fused is a negative regulator of sonic hedgehog signaling and alters the subcellular distribution of Gli1. Curr Biol. 1999;9:1119–22. [PubMed: 10531011]
- 71.
- Pearse IInd RV, Collier LS, Scott MP. et al. Vertebrate homologs of Drosophila suppressor of fused interact with the gli family of transcriptional regulators. Dev Biol. 1999;212:323–36. [PMC free article: PMC4530617] [PubMed: 10433824]
- 72.
- Stone DM, Murone M, Luoh S. et al. Characterization of the human suppressor of fused, a negative regulator of the zinc-finger transcription factor Gli. J Cell Sci. 1999;112:4437–48. [PubMed: 10564661]
- 73.
- Dahlen A, Fletcher CD, Mertens F. et al. Activation of the GLI oncogene through fusion with the beta-actin gene (ACTB) in a group of distinctive pericytic neoplasms: Pericytoma with t(7;12). Am J Pathol. 2004;164:1645–53. [PMC free article: PMC1615655] [PubMed: 15111311]
- 74.
- Oro AE, Higgins KM, Hu Z. et al. Basal cell carcinomas in mice overexpressing sonic hedgehog. Science. 1997;276:817–21. [PubMed: 9115210]
- 75.
- Nilsson M, Unden AB, Krause D. et al. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc Natl Acad Sci USA. 2000;97:3438–3443. [PMC free article: PMC16258] [PubMed: 10725363]
- 76.
- Oro AE, Higgins K. Hair cycle regulation of Hedgehog signal reception. Dev Biol. 2003;255:238–48. [PubMed: 12648487]
- 77.
- Grachtchouk V, Grachtchouk M, Lowe L. et al. The magnitude of hedgehog signaling activity defines skin tumor phenotype. EMBO J. 2003;22:2741–51. [PMC free article: PMC156767] [PubMed: 12773389]
- 78.
- Aszterbaum M, Epstein J, Oro A. et al. Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med. 1999;5:1285–1291. [PubMed: 10545995]
- 79.
- Grachtchouk M, Mo R, Yu S. et al. Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet. 2000;24:216–217. [PubMed: 10700170]
- 80.
- Sheng H, Goich S, Wang A. et al. Dissecting the oncogenic potential of Gli2: Deletion of an NH(2)-terminal fragment alters skin tumor phenotype. Cancer Res. 2002;62:5308–16. [PubMed: 12235001]
- 81.
- Hutchin ME, Kariapper MS, Grachtchouk M. et al. Sustained Hedgehog signaling is required for basal cell carcinoma proliferation and survival: Conditional skin tumorigenesis recapitulates the hair growth cycle Genes Dev 2004 [Epub ahead of print] [PMC free article: PMC545881] [PubMed: 15625189]
- 82.
- Goodrich LV, Milenkovic L, Higgins KM. et al. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. [PubMed: 9262482]
- 83.
- Hahn H, Wojnowski L, Zimmer AM. et al. Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. Nat Med. 1998;4:619–622. [PubMed: 9585239]
- 84.
- St-Jacques B, Dassule HR, Karavanova I. et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–68. [PubMed: 9768360]
- 85.
- Chiang C, Swan RZ, Grachtchouk M. et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev Biol. 1999;205:1–9. [PubMed: 9882493]
- 86.
- Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. [PubMed: 10375501]
- 87.
- Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–448. [PubMed: 10226030]
- 88.
- Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. [PubMed: 10027293]
- 89.
- Palma V, Ruiz i Altaba A. Hedgehog-GLI signaling regulates the behavior of cells with stem cell properties in the developing neocortex. Development. 2004;131:337–345. [PubMed: 14681189]
- 90.
- Lai K, Kaspar BK, Gage FH. et al. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. [PubMed: 12469128]
- 91.
- Machold R, Hayashi S, Rutlin M. et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. [PubMed: 12971894]
- 92.
- Palma V, Lim DA, Dahmane N. et al. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. [PMC free article: PMC1431583] [PubMed: 15604099]
- 93.
- Rowitch DH, S-Jacques B, Lee SM. et al. Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J Neurosci. 1999;19:8954–65. [PMC free article: PMC6782773] [PubMed: 10516314]
- 94.
- Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–72. [PubMed: 10821773]
- 95.
- van den Brink GR, Hardwick JC, Tytgat GN. et al. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology. 2001;121:317–28. [PubMed: 11487541]
- 96.
- Wang LC, Nassir F, Liu ZY. et al. Disruption of hedgehog signaling reveals a novel role in intestinal morphogenesis and intestinal-specific lipid metabolism in mice. Gastroenterology. 2002;122:469–82. [PubMed: 11832461]
- 97.
- Niemann C, Unden AB, Lyle S. et al. Indian hedgehog and beta-catenin signaling: Role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11873–80. [PMC free article: PMC304101] [PubMed: 12917489]
- 98.
- Lapidot T, Sirard C, Vormoor J. et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. [PubMed: 7509044]
- 99.
- Dick JE. Stem cells: Self-renewal writ in blood. Nature. 2003;423:231–3. [PubMed: 12748623]
- 100.
- Reya T, Morrison SJ, Clarke MF. et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. [PubMed: 11689955]
- 101.
- Mintz B, Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci USA. 1975;72:3585–9. [PMC free article: PMC433040] [PubMed: 1059147]
- 102.
- Papaioannou VE, McBurney MW, Gardner RL. et al. Fate of teratocarcinoma cells injected into early mouse embryos. Nature. 1975;258:70–73. [PubMed: 1186881]
- 103.
- Illmensee K, Mintz B. Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc Natl Acad Sci USA. 1976;73:549–53. [PMC free article: PMC335947] [PubMed: 1061157]
- 104.
- Ignatova TN, Kukekov VG, Laywell ED. et al. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. [PubMed: 12203386]
- 105.
- Al-Hajj M, Wicha MS, Benito-Hernandez A. et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–8. [PMC free article: PMC153034] [PubMed: 12629218]
- 106.
- Singh SK, Clarke ID, Terasaki M. et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed: 14522905]
- 107.
- Singh SK, Hawkins C, Clarke ID. et al. Identification of human brain tumor initiating cells Nature 2004432 396-401. [PubMed: 15549107]
- 108.
- Hemmati HD, Nakano I, Lazareff JA. et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA. 2003;100:15178–83. [PMC free article: PMC299944] [PubMed: 14645703]
- 109.
- Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA. 2004;101:781–6. [PMC free article: PMC321758] [PubMed: 14711994]
- 110.
- Galli R, Binda E, Orfanelli U. et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–21. [PubMed: 15466194]
- 111.
- Williams JA, Guicherit OM, Zaharian BI. et al. Identification of a small molecule inhibitor of the hedgehog signaling pathway: Effects on basal cell carcinoma-like lesions. Proc Natl Acad Sci USA. 2003;100:4616–21. [PMC free article: PMC153604] [PubMed: 12679522]
- 112.
- Berman DM, Karhadkar SS, Hallahan AR. et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–1561. [PubMed: 12202832]
- 113.
- Thayer SP, di Magliano MP, Heiser PW. et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. [PMC free article: PMC3688051] [PubMed: 14520413]
- 114.
- Kayed H, Kleeff J, Keleg S. et al. Indian hedgehog signaling pathway: Expression and regulation in pancreatic cancer. Int J Cancer. 2004;110:668–76. [PubMed: 15146555]
- 115.
- Watkins DN, Berman DM, Burkholder SG. et al. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–317. [PubMed: 12629553]
- 116.
- Berman DM, Karhadkar SS, Maitra A. et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. [PubMed: 14520411]
- 117.
- Sanchez P, Hernandez AM, Stecca B. et al. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci USA. 2004;101:12561–12566. [PMC free article: PMC514658] [PubMed: 15314219]
- 118.
- Karhadkar SS, Steven Bova G, Abdallah N. et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. [PubMed: 15361885]
- 119.
- Sheng T, Li C, Zhang X. et al. Activation of the hedgehog pathway in advanced prostate cancer. Mol Cancer. 2004;3:29. [PMC free article: PMC524523] [PubMed: 15482598]
- 120.
- Fan l, Pepicelli CV, Dibble CC. et al. Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology. 145:3961–3970. [PubMed: 15132968]
- 121.
- Kubo M, Nakamura M, Tasaki A. et al. Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer Res. 2004;64:6071–4. [PubMed: 15342389]
- 122.
- Stein U, Eder C, Karsten U. et al. GLI gene expression in bone and soft tissue sarcomas of adult patients correlates with tumor grade. Cancer Res. 1999;59:1890–5. [PubMed: 10213497]
- 123.
- Kumamoto H, Ohki K, Ooya K. Expression of Sonic hedgehog (SHH) signaling molecules in ameloblastomas. J Oral Pathol Med. 2004;33:185–90. [PubMed: 15128061]
- 124.
- Katayam M, Yoshida K, Ishimori H. et al. Patched and smoothened mRNA expression in human astrocytic tumors inversely correlates with histological malignancy. J Neurooncol. 2002;59:107–15. [PubMed: 12241103]
- 125.
- Nishimaki H, Kasai K, Kosaki K-C. et al. A role of activated Sonic hedgehog signaling for the cellular proliferation of oral squamous cell carcinoma cell line. Biochem Biophys Res Commun. 2004;314:313–320. [PubMed: 14733907]
- 126.
- Wuyts W, Van Hul W, Wauters J. et al. Positional cloning of a gene involved in hereditary multiple exostoses. Hum Mol Genet. 1996;5:1547–57. [PubMed: 8894688]
- 127.
- Levy P, Vidaud D, Leroy K. et al. Molecular profiling of malignant peripheral nerve sheath tumors associated with neurofibromatosis type 1, based on large-scale real-time RT-PCR. Mol Cancer. 2004;3:20. [PMC free article: PMC493279] [PubMed: 15255999]
- 128.
- Hamed S, LaRue H, Hovington H. et al. Accelerated induction of bladder cancer in patched heterozygous mutant mice. Cancer Res. 2004;64:1938–42. [PubMed: 15026327]
- 129.
- Qualtrough D, Buda A, Gaffield W. et al. Hedgehog signalling in colorectal tumour cells: Induction of apoptosis with cyclopamine treatment. Int J Cancer. 2004;110:831–7. [PubMed: 15170664]
- 130.
- Oniscu A, James RM, Morris RG. et al. Expression of Sonic hedgehog pathway genes is altered in colonic neoplasia. J Pathol. 2004;203:909–17. [PubMed: 15258993]
- 131.
- van den Brink GR, Bleuming SA, Hardwick JC. et al. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat Genet. 2004;36:277–82. [PubMed: 14770182]
- 132.
- Zhu Y, James RM, Peter A. et al. Functional Smoothened is required for expression of GLI3 in colorectal carcinoma cells. Cancer Lett. 2004;207:205–14. [PubMed: 15072830]
- 133.
- Ohki K, Kumamoto H, Ichinohasama R. et al. PTC gene mutations and expression of SHH, PTC, SMO, and GLI-1 in odontogenic keratocysts. Int J Oral Maxillofac Surg. 2004;33:584–92. [PubMed: 15308259]
- 134.
- Olsen CL, Hsu PP, Glienke J. et al. Hedgehog-interacting protein is highly expressed in endothelial cells but down-regulated during angiogenesis and in several human tumors. BMC Cancer. 2004;4:43. [PMC free article: PMC512291] [PubMed: 15294024]
- 135.
- Chuang PT, McMahon AP. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature. 1999;397:617–21. [PubMed: 10050855]
- 136.
- Taipale J, Chen JK, Cooper MK. et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–9. [PubMed: 10984056]
- 137.
- Park HL, Bai C, Platt KA. et al. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development. 2000;127:1593–1605. [PubMed: 10725236]
- 138.
- Weiner HL, Bakst R, Hurlbert MS. et al. Induction of medulloblastomas in mice by sonic hedgehog, independent of Gli1. Cancer Res. 2002;62:6385–9. [PubMed: 12438220]
- 139.
- Chin L, Tam A, Pomerantz J. et al. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400:468–72. [PubMed: 10440378]
- 140.
- Wong AK, Chin L. An inducible melanoma model implicates a role for RAS in tumor maintenance and angiogenesis. Cancer Metastasis Rev. 2000;19:121–9. [PubMed: 11191050]
- 141.
- Fisher GH, Wellen SL, Klimstra D. et al. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 2001;15:3249–62. [PMC free article: PMC312852] [PubMed: 11751631]
- 142.
- Keeler RF, Binns W. Teratogenic compounds of Veratrum californicum (Durand). V. Comparison of cyclopian effects of steroidal alkaloids from the plant and structurally related compounds from other sources. Teratology. 1968;1:5–10. [PubMed: 5696817]
- 143.
- Keeler RF. Comparison of the teratogenicity in rats of certain potato-type alkaloids and the veratrum teratogen cyclopamine. Lancet. 1973;1:1187–8. [PubMed: 4123576]
- 144.
- Keeler RF. Teratogenic effects of cyclopamine and jervine in rats, mice and hamsters. Proc Soc Exp Biol Med. 1975;149:302–6. [PubMed: 1144444]
- 145.
- Keeler RF. Cyclopamine and related steroidal alkaloid teratogens: Their occurrence, structural relationship, and biologic effects. Lipids. 1978;13:708–15. [PubMed: 723484]
- 146.
- Keeler RF. Teratogenic compounds of Veratrum californicum (Durand) X. Cyclopia in rabbits produced by cyclopamine. Teratology. 1970;3:175–80. [PubMed: 4986632]
- 147.
- Keeler RF, Baker DC. Oral, osmotic minipump, and intramuscular administration to sheep of the Veratrum alkaloid cyclopamine. Proc Soc Exp Biol Med. 1989;192:153–6. [PubMed: 2813445]
- 148.
- Chiang C, Litingtung Y, Lee E. et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–13. [PubMed: 8837770]
- 149.
- Belloni E, Muenke M, Roessler E. et al. Identification of Sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat Genet. 1996;14:353–356. [PubMed: 8896571]
- 150.
- Roessler E, Belloni E, Gaudenz K. et al. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat Genet. 1996;14:357–360. [PubMed: 8896572]
- 151.
- Incardona JP, Gaffield W, Kapur RP. et al. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development. 1998;125:3553–3562. [PubMed: 9716521]
- 152.
- Cooper MK, Porter JA, Young KE. et al. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–1607. [PubMed: 9616123]
- 153.
- Chen JK, Taipale J, Cooper MK. et al. Inhibition of Hedgehog signal by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–2748. [PMC free article: PMC187469] [PubMed: 12414725]
- 154.
- Chen JK, Taipale J, Young KE. et al. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA. 2002;99:14071–6. [PMC free article: PMC137838] [PubMed: 12391318]
- 155.
- Frank-Kamenetsky M, Zhang XM, Bottega S. et al. Small-molecule modulators of Hedgehog signaling: Identification and characterization of Smoothened agonists and antagonists. J Biol. 2002;1:10. [PMC free article: PMC137065] [PubMed: 12437772]
- 156.
- Ericson J, Morton S, Kawakami A. et al. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–73. [PubMed: 8929535]
- 157.
- Vogt A, Chuang PT, Hebert J. et al. Immunoprevention of basal cell carcinomas with recombinant hedgehog-interacting protein. J Exp Med. 2004;199:753–61. [PMC free article: PMC2212732] [PubMed: 15024045]
- 158.
- Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9:4227–39. [PubMed: 14519650]
- 159.
- Kelland LR. Of mice and men: Values and liabilities of the athymic nude mouse model in anticancer drug development. Eur J Cancer. 2004;40:827–36. [PubMed: 15120038]
- 160.
- Wetmore C, Eberhart DE, Curran T. Loss of p53 but not ARF accelerates medulloblastoma in mice heterozygous for patched. Cancer Res. 2001;61:513–6. [PubMed: 11212243]
- 161.
- Romer JT, Kimura H, Magdaleno S. et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/-)p53(-/-) mice. Cancer Cell. 2004;6:229–240. [PubMed: 15380514]
- 162.
- Sanchez P, Ruiz i Altaba A. In vivo inhibition of endogenous brain tumors through systemic interference of Hedgehog signaling in mice. Mech Dev. 2005;122:223–230. [PubMed: 15652709]
- 163.
- Bellemeyer A, Krase J, Lindgren J. et al. The protooncogene c-Myc is an essential regulator of neural crest formation in Xenopus. Dev Cell. 2003;4:827–839. [PubMed: 12791268]
- 164.
- Cano A, Perez-Moreno MA, Rodrigo I. et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. [PubMed: 10655586]
- 165.
- Kenney AM, Widlund HR, Rowitch DH. Hedgehog and PI-3 kinase signaling converge on Nmyc1 to promote cell cycle progression in cerebellar neuronal precursors. Development. 2004;131:217–28. [PubMed: 14660435]
- 166.
- Kenney AM, Cole MD, Rowitch DH. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development. 2003;130:15–28. [PubMed: 12441288]
- 167.
- Louro ID, Bailey EC, Li X. et al. Comparative gene expression profile analysis of GLI and c-MYC in an epithelial model of malignant transformation. Cancer Res. 2002;62:5867–73. [PubMed: 12384550]
- 168.
- Tabs S, Avci O. Induction of the differentiation and apoptosis of tumor cells in vivo with efficiency and selectivity. Eur J Dermatol. 2004;14:96–102. [PubMed: 15196999]
- 169.
- Tas S, Avci O. Rapid clearance of psoriatic skin lesions induced by topical cyclopamine. A preliminary proof of concept study. Dermatology. 2004;209:126–131. [PubMed: 15316166]
- 170.
- Brewster R, Mullor JL, Ruiz i Altaba A. Gli2 functions in FGF signaling during antero-posterior patterning. Development. 2000;127:4395–405. [PubMed: 11003839]
- 171.
- Ruiz i Altaba A. Catching a Gli-mpse of Hedgehog. Cell. 1997;90:193–6. [PubMed: 9244291]
- 172.
- Ruiz i Altaba A. Combinatorial Gli gene function in floor plate and neuronal inductions by Sonic hedgehog. Development. 1998;125:2203–12. [PubMed: 9584120]
- 173.
- Hammerschmidt M, Bitgood MJ, McMahon AP. Protein kinase A is a common negative regulator of Hedgehog signaling in the vertebrate embryo. Genes Dev. 1996;10:647–58. [PubMed: 8598293]
- 174.
- Chen Y, Gallaher N, Goodman RH. et al. Protein kinase A directly regulates the activity and proteolysis of cubitus interruptus. Proc Natl Acad Sci USA. 1998;95:2349–54. [PMC free article: PMC19341] [PubMed: 9482888]
- 175.
- Ohlmeyer JT, Kalderon D. Hedgehog stimulates maturation of Cubitus interruptus into a labile transcriptional activator. Nature. 1998;396:749–53. [PubMed: 9874371]
- 176.
- Wang G, Wang B, Jiang J. Protein kinase A antagonizes Hedgehog signaling by regulating both the activator and repressor forms of Cubitus interruptus. Genes Dev. 1999;13:2828–37. [PMC free article: PMC317143] [PubMed: 10557210]
- 177.
- Jia J, Amanai K, Wang G. et al. Shaggy/GSK3 antagonizes Hedgehog signalling by regulating Cubitus interruptus. Nature. 2002;416:548–52. [PubMed: 11912487]
- 178.
- Jia J, Tong C, Wang B. et al. Hedgehog signalling activity of Smoothened requires phosphorylation by protein kinase A and casein kinase I. Nature. 2004;432:1045–50. [PubMed: 15616566]
- 179.
- Price MA, Kalderon D. Proteolysis of cubitus interruptus in Drosophila requires phosphorylation by protein kinase A. Development. 1999;126:4331–9. [PubMed: 10477300]
- 180.
- Price MA, Kalderon D. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by Glycogen Synthase Kinase 3 and Casein Kinase 1. Cell. 2002;108:823–35. [PubMed: 11955435]
- 181.
- Apionishev S, Katanayeva NM, Marks SA. et al. Drosophila Smoothened phosphorylation sites essential for Hedgehog signal transduction Nat Cell Biol 2004 [Epub ahead of print] [PubMed: 15592457]
- 182.
- Chen W, Ren XR, Nelson CD. et al. Activity-dependent internalization of smoothened mediated by beta-arrestin 2 and GRK2. Science. 2004;306:2257–60. [PubMed: 15618519]
- 183.
- Ogden SK, Ascano Jr M, Stegman MA. et al. Regulation of Hedgehog signaling: A complex story. Biochem Pharmacol. 2004;67:805–14. [PMC free article: PMC3659400] [PubMed: 15104233]
- 184.
- Mao J, Maye P, Kogerman P. et al. Regulation of Gli1 transcriptional activity in the nucleus by Dyrk1. J Biol Chem. 2002;277:35156–61. [PubMed: 12138125]
- 185.
- Huangfu D, Liu A, Rakeman AS. et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–7. [PubMed: 14603322]
- 186.
- Brewster R, Lee J, Ruiz i Altaba A. Gli/Zic factors pattern the neural plate by defining domains of cell differentiation. Nature. 1998;393:579–83. [PubMed: 9634234]
- 187.
- Koyabu Y, Nakata K, Mizugishi K. et al. Physical and functional interactions between Zic and Gli proteins. J Biol Chem. 2001;276:6889–92. [PubMed: 11238441]
- 188.
- Di Marcotullio L, Ferretti E, De Smaele E. et al. REN(KCTD11) is a suppressor of Hedgehog signaling and is deleted in human medulloblastoma. Proc Natl Acad Sci USA. 2004;101:10833–8. [PMC free article: PMC490020] [PubMed: 15249678]
- 189.
- Wolff C, Roy S, Lewis KE. et al. Iguana encodes a novel zinc-finger protein with coiled-coil domains essential for Hedgehog signal transduction in the zebrafish embryo. Genes Dev. 2004;18:1565–76. [PMC free article: PMC443519] [PubMed: 15198976]
- 190.
- Sekimizu K, Nishioka N, Sasaki H. et al. The zebrafish iguana locus encodes Dzip1, a novel zinc-finger protein required for proper regulation of Hedgehog signaling. Development. 2004;131:2521–32. [PubMed: 15115751]
- 191.
- Eggenschwiler JT, Espinoza E, Anderson KV. Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature. 2001;412:194–8. [PubMed: 11449277]
- 192.
- Bulgakov OV, Eggenschwiler JT, Hong DH. et al. FKBP8 is a negative regulator of mouse sonic hedgehog signaling in neural tissues. Development. 2004;131:2149–59. [PubMed: 15105374]
- 193.
- Cheng SY, Bishop JM. Suppressor of Fused represses Gli-mediated transcription by recruiting the SAP18-mSin3 corepressor complex. Proc Natl Acad Sci USA. 2002;99:5442–7. [PMC free article: PMC122788] [PubMed: 11960000]
- 194.
- Merchant M, Vajdos FF, Ultsch M. et al. Suppressor of fused regulates Gli activity through a dual binding mechanism. Mol Cell Biol. 2004;24:8627–41. [PMC free article: PMC516763] [PubMed: 15367681]
- 195.
- Pomeroy SL, Tamayo P, Gaasenbeek M. et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415:436–42. [PubMed: 11807556]
- 196.
- Mullor JL, Dahmane N, Sun T. et al. Wnt signals are targets and mediators of Gli function. Curr Biol. 2001;11:769–73. [PubMed: 11378387]
- 197.
- Nicolas M, Wolfer A, Raj K. et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–21. [PubMed: 12590261]
- 198.
- Ke Z, Emelyanov A, Lim SE. et al. Expression of a novel zebrafish zinc finger gene, gli2b, is affected in Hedgehog and Notch signaling related mutants during embryonic development Dev Dyn 2004. [Epub ahead of print] [PubMed: 15614761]
- 199.
- Okamoto M. Simultaneous demonstration of lens regeneration from dorsal iris and tumour production from ventral iris in the same newt eye after carcinogen administration. Differentiation. 1997;61:285–92. [PubMed: 9342839]
- 200.
- Zuñiga A, Zeller R. Gli3 (Xt) and formin (ld) participate in the positioning of the polarising region and control of posterior limb-bud identity. Development. 1999;126:13–21. [PubMed: 9834182]
- 201.
- Zakany J, Kmita M, Duboule D. A dual role for Hox genes in limb anterior-posterior asymmetry. Science. 2004;304:1669–72. [PubMed: 15192229]
- 202.
- Chen Y, Knezevic V, Ervin V. et al. Direct interaction with Hoxd proteins reverses Gli3-repressor function to promote digit formation downstream of Shh. Development. 2004;131:2339–47. [PubMed: 15102708]
- 203.
- Joulia L, Bourbon H-M, Cribbs DL. Homeotic proboscipedia function modulates hedgehog-mediated organizer activity to pattern adult Drosophila mouthparts. Dev Biol. 2005;278:496–505. [PubMed: 15680366]
- 204.
- Ruiz i Altaba A, Melton DA. Interaction between peptide growth factors and homoeobox genes in the establishment of antero-posterior polarity in frog embryos. Nature. 1989;341:33–8. [PubMed: 2570357]
- 205.
- Kmita M, Duboule D. Organizing axes in time and space; 25 years of colinear tinkering. Science. 2003;301:331–3. [PubMed: 12869751]
- 206.
- Gaufo GO, Flodby P, Capecchi MR. Hoxb1 controls effectors of sonic hedgehog and Mash1 signaling pathways. Development. 2000;127:5343–54. [PubMed: 11076756]
- 207.
- Amsellem S, Pflumio F, Bardinet D. et al. Ex vivo expansion of human hematopoietic stem cells by direct delivery of the HOXB4 homeoprotein. Nat Med. 2003;9:1423–7. [PubMed: 14578882]
- 208.
- Krosl J, Austin P, Beslu N. et al. In vitro expansion of hematopoietic stem cells by recombinant TAT-HOXB4 protein. Nat Med. 2003;9:1428–32. [PubMed: 14578881]
- 209.
- Zhang Y, Zhao X, Hu Y. et al. Msx1 is required for the induction of Patched by Sonic hedgehog in the mammalian tooth germ. Dev Dyn. 1999;215:45–53. [PubMed: 10340755]
- 210.
- Cillo C, Cantile M, Faiella A. et al. Homeobox genes in normal and malignant cells. J Cell Physiol. 2001;188:161–9. [PubMed: 11424082]
- 211.
- Abate-Shen C. Homeobox genes and cancer: New OCTaves for an old tune. Cancer Cell. 2003;4:329–30. [PubMed: 14667497]
- 212.
- Galis F. Why do almost all mammals have seven cervical vertebrae? Developmental constraints, Hox genes, and cancer. J Exp Zool. 1999;285:19–26. [PubMed: 10327647]
- 213.
- Franco PG, Paganelli AR, Lopez SL. et al. Functional association of retinoic acid and hedgehog signaling in Xenopus primary neurogenesis. Development. 1999;126:4257–65. [PubMed: 10477294]
- 214.
- McKay RD. Stem cell biology and neurodegenerative disease. Philos Trans R Soc Lonon B Biol Sci. 2004;359:851–856. [PMC free article: PMC1693367] [PubMed: 15293812]
- 215.
- Miao N, Wang M, Ott JA. et al. Sonic hedgehog promotes the survival of specific CNS neuron populations and protects these cells from toxic insult In vitro. J Neurosci. 1997;17:5891–9. [PMC free article: PMC6573190] [PubMed: 9221786]
- 216.
- Hurtado-Lorenzo A, Millan E, Gonzalez-Nicolini V. et al. Differentiation and transcription factor gene therapy in experimental parkinson's disease: Sonic hedgehog and Gli-1, but not Nurr-1, protect nigrostriatal cell bodies from 6-OHDA-induced neurodegeneration. Mol Ther. 2004:507–24. [PMC free article: PMC1479772] [PubMed: 15336651]
- 217.
- Suwelack D, Hurtado-Lorenzo A, Millan E. et al. Neuronal expression of the transcription factor Gli1 using the Talpha1 alpha-tubulin promoter is neuroprotective in an experimental model of Parkinson's disease. Gene Ther. 2004;11:1742–52. [PMC free article: PMC1249480] [PubMed: 15573088]
- 218.
- Mancuso M, Pazzaglia S, Tanori M. et al. Basal cell carcinoma and its development: Insights from radiation-induced tumors in Ptch1-deficient mice. Cancer Res. 2004;64:934–941. [PubMed: 14871823]
- 219.
- Pazzaglia S, Mancuso M, Atkinson MJ. et al. High incidence of medulloblastoma following X-Ray-irradiation of newborn Ptc1 heterozygous mice. Oncogene. 2002;21:7580–7584. [PubMed: 12386820]
- 220.
- Regl G, Kasper M, Schnidar H. et al. Activation of the Bcl2 promoter in response to Hedgehog/ GLI signal transduction is predominantly mediated by Gli2. Cancer Res. 2004;64:7724–7731. [PubMed: 15520176]
- 221.
- Rao G, Pedone CA, Coffin CM. et al. c-Myc enhances sonic hedgehog-induced medulloblastoma formation from nestin-expressing neural progenitors in mice. Neoplasia. 2003;5:198–204. [PMC free article: PMC1502413] [PubMed: 12869303]
- 222.
- Rao G, Pedone CA, Valle LD. et al. Sonic hedgehog and insulin-like growth factor signaling synergize to induce medulloblastoma formation from nestin-expressing neural progenitors in mice. Oncogene. 2004;23:6156–62. [PubMed: 15195141]
- 223.
- Callahan CA, Ofstad T, Horng L. et al. MIM/BEG4, a Sonic hedgehog-responsive gene that potentiates Gli-dependent transcription. Genes Dev. 2004;18(22):2724–9. [PMC free article: PMC528890] [PubMed: 15545630]
- 224.
- Athar M, Li C, Tang X. et al. Inhibition of smoothened signaling prevents ultraviolet B-induced basal cell carcinomas through regulation of Fas expression and apoptosis. Cancer Res. 2004;64(20):7545–52. [PubMed: 15492281]
- 225.
- Pu Y, Huang L, Prins GS. Sonic hedgehog-patched Gli signaling in the developing rat prostate gland: lobe-specific suppression by neonatal estrogens reduces ductal growth and branching. Dev Biol. 2004;273(2):257–75. [PMC free article: PMC2978068] [PubMed: 15328011]
- 226.
- Alberi L, Sgado P, Simon HH. Engrailed genes are cell-autonomously required to prevent apoptosis in mesencephalic dopaminergic neurons. Development. 2004;131(13):3229–36. [PubMed: 15175251]
- 227.
- Prochiantz A. Protein transduction: from physiology to technology and vice versa. Adv Drug Deliv Rev. 2005;57(4):491–3. [PubMed: 15722159]
- 228.
- Johnston JJ, Olivos-Glander I, Killoran C. et al. Molecular and clinical analyses of Greig cephalopolysyndactyly and Pallister-Hall syndromes: robust phenotype prediction from the type and position of GLI3 mutations. Am J Hum Genet. 2005;76(4):609–22. [PMC free article: PMC1199298] [PubMed: 15739154]
Publication Details
Author Information and Affiliations
Authors
Ariel Ruiz i Altaba*.Affiliations
Copyright
Publisher
Landes Bioscience, Austin (TX)
NLM Citation
Ruiz i Altaba A. How the Hedgehog Outfoxed the Crab: Interference with HEDGEHOG-GLI Signaling as Anti-Cancer Therapy? In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.