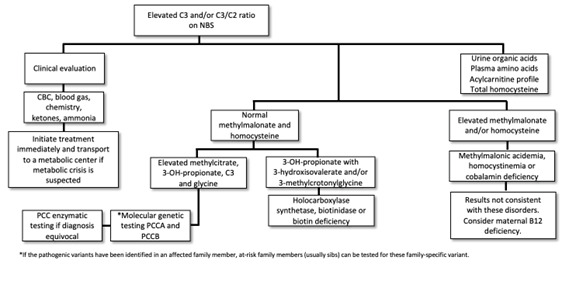
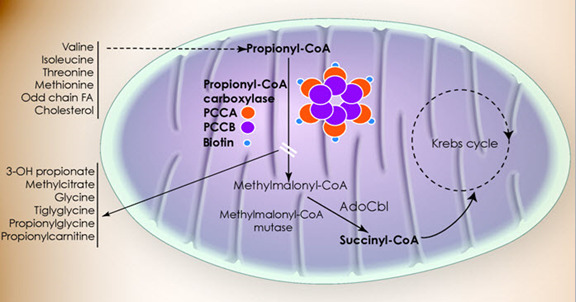
Propionic acidemia (PA) presents with a wide spectrum of symptoms and age of onset. The onset of symptoms in PA varies depending on several factors, including residual enzymatic activity, intake of propiogenic precursors, and the occurrence of catabolic stressors.
Neonatal-Onset PA
A common presentation of PA in the neonatal period is characterized by a healthy newborn with poor feeding and decreased arousal in the first few days of life, followed by progressive encephalopathy of unexplained origin. Most individuals eventually diagnosed with PA become symptomatic in the first weeks of life, with 50%-60% exhibiting clinical signs at the time of the newborn screen (NBS) report. Without prompt diagnosis and management, neonates can develop progressive encephalopathy manifesting as lethargy, seizures, or coma that can result in death (see Table 2).
Table 2.
Propionic Acidemia: Findings During Initial Metabolic Crisis
View in own window
| Symptoms | Prevalence 1, 2 |
|---|
| Feeding difficulties | 42%-87% |
| Poor weight gain and growth | 58%-71% |
| Vomiting | 45%-61% |
| Hypotonia | 47%-69% |
| Somnolence | 35%-70% |
| Coma | 12%-26% |
| Seizures | 12%-60% |
| Tachypnea | 29%-48% |
| Hypothermia | 20% |
| Hyperammonemia 3 | 41%-97% |
| Metabolic acidosis | 55%-73% |
| Hypoglycemia | 19%-20% |
| Anemia | 8%-57% |
| Leukopenia | 25%-52% |
| Thrombocytopenia | 8%-23% |
- 1.
The differences in the reported prevalence of findings may reflect variable sizes of the cohorts, age of the last evaluation, length of follow up, differences in therapeutic approaches, availability, turnaround time and sensitivity of NBS, screening method (NBS vs selective metabolic screen), overlap of affected individuals in the reported cohorts, and ascertainment and recall bias.
- 2.
- 3.
The mean plasma ammonia levels in people with neonatal-onset PA was reported to vary between 207 and 697 μmol/L [Grünert et al 2012, Kölker et al 2015a]. Reported plasma ammonia levels fall between 86 and 3,377 μmol/L [Kölker et al 2015a]. In contrast to urea cycle disorders, hyperammonemia in PA is usually accompanied by low or normal level of glutamine.
Following initial clinical and biochemical stabilization, individuals with neonatal-onset PA may experience metabolic decompensations and can develop a range of symptoms affecting different organ systems.
Metabolic decompensations. Individuals affected by PA can develop episodic metabolic decompensations, especially in the first years of life. Metabolic acidosis, hyperammonemia, pancreatitis, metabolic strokes, cardiomyopathy, bone marrow suppression, seizures, and encephalopathy can accompany acutely deranged metabolism.
These episodes can be life-threatening and are often precipitated by illnesses, infections, surgery, or any physiologic stress that can trigger a catabolic state.
Infectious complications (e.g., sepsis or bacterial meningitis) often accompany metabolic crises and are the major contributors to mortality.
Growth. Linear growth delay and deceleration of the head circumference may become evident with age and can be seen in both earlier- and later-onset groups [Kölker et al 2015b, Saleemani et al 2021]. Poor growth may be exacerbated by malnutrition secondary to feeding difficulties, recurrent emesis, excessive protein restriction, and potentially iatrogenic amino acid imbalances [Manoli et al 2016].
Neurologic manifestations include developmental delay, developmental regression, intellectual disability, seizures, hypotonia, spasticity, and movement disorders [Nizon et al 2013, Shchelochkov et al 2024].
Seizures were reported in 13%-53% and EEG abnormalities in 40%-63% of individuals with PA [
Karimzadeh et al 2014,
AlGhamdi et al 2018]. Reported types of seizures include infantile spasms, tonic-clonic, tonic, myoclonic, atonic, absence, and focal. Seizures were one of the presenting features of the initial metabolic episode in 12%-26% of affected individuals [
Kölker et al 2015b].
Basal ganglia and brain MRI findings. Individuals with PA are at high risk for basal ganglia lesions, especially during episodes of acute encephalopathy or metabolic instability [
Nizon et al 2013,
Karimzadeh et al 2014].
Basal ganglia changes seen in 7%-56% of individuals may be preceded by an acute "stroke-like" episode and manifest as altered mental status, dystonia, choreoathetosis, or hemiplegia.
A unique pattern of cortical and subcortical diffusion restriction can be seen on brain MRI in some affected individuals [
Pfeifer et al 2018].
Other brain MRI findings can include delayed myelination, white matter changes, cerebral atrophy, cerebellar atrophy, and cerebellar hemorrhage.
Clinically unstable individuals appear to be at higher risk of developing brain abnormalities. In a study of 17 individuals with PA who had clinical seizures, all had abnormal MRI findings and a history of more than ten metabolic decompensations [
Haberlandt et al 2009].
Magnetic resonance spectroscopy (MRS) can reveal decreased myoinositol and N-acetylaspartate and abnormal Glx (glutamine, glutamate, and gamma-aminobutyric acid) peaks in the basal ganglia.
Intellectual disability. Developmental delays and neurologic dysfunction can be documented even in individuals without known episodes of hyperammonemia or ketoacidosis [
Schreiber et al 2012]. The prevalence of intellectual disability varies between 32% and 76% depending on the reported cohort [
Pena & Burton 2012,
Shchelochkov et al 2024].
Cardiomyopathy has been recognized as a common complication of PA. Both dilated and hypertrophic cardiomyopathy have been observed. The reported prevalence varies between 7% and 39% in PA cohorts [Romano et al 2010, Kovacevic et al 2020].
Early clinical manifestations of cardiomyopathy include increased fatigue, tachypnea, hepatomegaly, hypotension, tachycardia, or bradycardia.
The age of PA diagnosis and frequency of metabolic decompensation do not correlate with presence/absence of cardiomyopathy in individuals with PA [
Romano et al 2010].
Cardiomyopathy can progress to cardiac failure and may be associated with sudden death.
Cardiac rhythm abnormalities. A prolonged QT interval is often detected in individuals with PA [Kölker et al 2015b]. This can be associated with syncope, arrhythmia, and cardiac arrest [Della Rossa et al 2022].
Gastrointestinal manifestations
Renal abnormalities. Chronic kidney disease is observed in half of individuals with PA and can lead to kidney failure and the need for kidney transplantation [Shchelochkov et al 2019].
Hematologic abnormalities. Although anemia, leukopenia, and thrombocytopenia are common, pancytopenia is seen less frequently, in 6%-15% of individuals [Kölker et al 2015b].
Immune system. Early retrospective data suggested a high frequency of recurrent infections, seen in 60%-80% of affected individuals. Factors predisposing to infectious complications are likely diverse and may include bone marrow suppression, immune dysfunction instigated by propionic acid metabolites, indwelling catheters (e.g., central lines), frequent hospitalizations, and potential nutritional deficiencies caused by dietary modification.
Hypogammaglobulinemia, B-cell lymphopenia, neutropenia, decreased CD4 and CD8 counts, decreased naïve T cells, and abnormal CD4/CD8 ratio have been described [Altun et al 2022]. Hypogammaglobulinemia, reported in as many as 15% of affected individuals, has required treatment with immunoglobulin in some cases [Pena & Burton 2012].
Ophthalmologic
manifestations. Eye findings include dyschromatopsia, optic nerve atrophy, scotomas, and abnormal electroretinogram and visual evoked potentials. In addition, optic tract and cortical abnormalities have been occasionally noted [Noval et al 2013, Arias et al 2014].
Optic neuropathy occurs in 11%-25% of affected individuals [Pena & Burton 2012, Martinez Alvarez et al 2016]. The onset of optic neuropathy can be acute or insidious; further deterioration can occur during metabolic decompensations triggered by infections or surgery [Martinez Alvarez et al 2016]. The mean age of diagnosis is approximately 13 years (range: 2-24 years) [Arias et al 2014, Martinez Alvarez et al 2016].
Hearing loss. Sensorineural hearing loss was reported in 1% and 13% in two large cohorts of individuals with PA [Grünert et al 2012, Kölker et al 2015b].
Musculoskeletal system. Severe osteopenia and osteoporosis have been described in adults with PA [Grünert et al 2012].
Dermatologic manifestations resembling acrodermatitis enteropathica are frequently associated with deficiency of essential amino acids, particularly isoleucine, which can be inadvertently overrestricted in the diet of persons with PA [Domínguez-Cruz et al 2011].
Other rare complications.
Isolated case reports describe clinical findings that could be causally associated with propionic acidemia but require further characterization: muscle lipidosis [de Baulny et al 2005]; myopathy [Martinez Alvarez et al 2016]; premature ovarian insufficiency [Lam et al 2011]; oligomenorrhea [Martín-Hernández et al 2009]; hypothyroidism [Vernon et al 2014, Martinez Alvarez et al 2016]; and parathyroid hormone resistance resolving after hemodialysis [Griffin et al 1996].