NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Wilt TJ, Shamliyan T, Taylor B, et al. Comparative Effectiveness of Therapies for Clinically Localized Prostate Cancer [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008 Feb. (Comparative Effectiveness Reviews, No. 13.)

Comparative Effectiveness of Therapies for Clinically Localized Prostate Cancer [Internet].
Show detailsTopic Development
The topic of this report and preliminary key questions were developed through a public process involving the public, the Scientific Resource Center (www.effectivehealthcare.ahrq.gov/aboutUS/contract.cfm) for the Effective Health Care program of AHRQ, and various stakeholder groups. Additional study, patient, intervention, and eligibility criteria, as well as outcomes, were refined and agreed upon through discussions between the Minnesota EPC, the Technical Expert Panel (TEP) members, our AHRQ Task Order Officer, and comments received by the public.
Literature Search and Review Strategy
To address questions 1, 2, and 4 we relied on several sources of data. First randomized controlled trials published through mid-September 2007 were identified using the Cochrane Library and the Cochrane Review Group in Prostate Diseases specialized registry. For health status and quality of life studies, a literature search was conducted on Ovid MEDLINE, using the search termsprostatic neoplasms,quality of life,QOL,HRQOL, andhealth status. The search was limited to English language randomized trials or large prospective U.S. observational studies published from 2000 to September 2007.
Because our search of RCTs yielded very few trials directly comparing the major treatment options, especially for PSA-detected prostate cancer, we reanalyzed results from a database primarily comprised of nonrandomized studies and previously extracted by our group (TJ Wilt principal contract recipient) under a separate prior contract with AUA for the American Urological Association Treatment Guidelines Panel for Clinically Localized Prostate Cancer. These data were used for development of the “Guidelines for the Management of Clinically Localized Prostate Cancer: 2007 update” 29 and subsequently provided to us as a raw database under a separate contract for additional analysis for this comparative effectiveness review. Studies were identified by the AUA by a series of four PubMed searches conducted by the AUA Guideline Panel between May 2001 and April 2004. This search captured articles published from 1991 through April 2004. Articles identified by the AUA search team and deemed eligible for the AUA Prostate Cancer Guideline were sent to the Minnesota EPC research team for additional evaluation, determination of eligibility, and study extraction. Articles were rejected if patients with higher stage disease were included in the study and the outcomes were not stratified by stage. The 592 articles meeting these inclusion criteria were retrieved for data extraction. An extraction form was developed that included patient characteristics, treatments, and outcomes data, such as the definition of biochemical progression used in the study, survival, disease-free survival, and progression to invasive disease. During the extraction process, articles again were scanned for relevance and were rejected if outcomes were not reported or stratified for clinically localized disease or if outcomes in fewer than 50 patients were reported. Upon completion, which included several quality assurance checks, data from 592 articles were extracted and entered into a Microsoft. Access© (Microsoft, Redmond, WA) database. Articles of cryotherapy, laparoscopic or robotic assisted prostatectomy, HIFU, proton beam radiation, and IMRT with or without imaging guidance, identified through Medline, contact with Endocare (a manufacturer of cryotherapy devices) and published between April 2004 and September 2007 were included because little published literature on these technologies was available for the AUA Guideline.
Questions 2 and 4 were addressed by reviewing RCTs for comparative effectiveness according to patient (age, race, comorbidities) or tumor characteristics (PSA, tumor stage, histologic grade, tumor risk strata). Any study from the AUA database or U.S. population-based studies that had outcomes stratified according to age or race was extracted, looking for comparative effectiveness between treatments according to these factors (rather than absolute effectiveness of an individual treatment). Due to the initial findings by the AUA Treatment Guideline Panel indicating poor methodologic design quality and reporting of outcomes from nonrandomized trials, our TEP members determined that an updated search for additional high quality evidence or inclusion of nonrandomized studies published after April 2004, was not indicated and would be biased in evaluating comparative effectiveness. They unanimously recommended against such an update. An updated case series assessing long-term outcomes of men in the United States managed with expectant management was included because little is known about the natural history of prostate cancer, especially stratified by patient's age and Gleason score.
Several strategies were used to assess the comparative effectiveness of treatments according to provider characteristics. The ideal method would be to analyze RCT evidence that examined how provider characteristics modified the effectiveness of different treatments. Because no randomized trials were found, we reviewed the evidence of heterogeneity in outcomes in multi center studies and possible subgroup analysis by provider characteristics. The third possible strategy was to review absolute risks of outcomes in different studies in relation to self-reported provider characteristics for possible comparisons across the studies. We excluded reports that only assessed self-reported provider volumes, training, the affiliation with medical schools, experience, and other characteristics. Search terms included MESH major headings of prostate cancer and prostatic neoplasm and were limited to human subjects and English language.
For question 3, the following databases were searched to identify reports of human studies published in English from 1992 to August 2006 (for volume outcome relationships we searched through September 2007): The National Library of Medicine via PubMed®; Cochrane Library; CDC Website; Catalog of U.S. Government Publications (U.S. GPO); LexisNexis™ Government Periodicals Index; and Digital Dissertations. The MeSH terms, key words, and its combinations are presented in Appendix A. The Analytic Framework (Figure 1) outlines the hypothesized relationships between the exposures (bold), outcomes (italic bold) , and effect modifiers (underlined) variables.
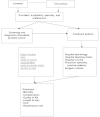
Figure
Figure 1. Analytic framework for Key Question 3.
Study Selection
Criteria for Selecting Studies for This Review (Table 2)
Types of studies. For questions 1, 2, and 4 randomized trials were included if the randomized treatment allocation was based on men with clinically localized disease and reported clinical outcomes separately for T1 and T2 disease. Since no randomized trials investigated the role of patient race or ethnicity on the efficacy and AEs associated with localized prostate cancer and its treatment, we were left with only observational studies. Studies from the AUA database that had outcomes stratified according to age, race, PSA, stage, or histology were extracted looking for comparative effectiveness between treatments according to these factors, (rather then absolute effectiveness of an individual treatment). We also included population based studies published through March 2007 evaluating watchful waiting for T1–T2 disease and containing at least 100 men because little is known about the natural history of prostate cancer. We included studies of treatment effectiveness, harms, and patient satisifaction from the Prostate Cancer Outcomes Study (PCOS) cohort study or the National Cancer Institutes Surveillance, Epidemiology, and End Results (SEER) program published through September 2007 because these were large, nationally representative studies that enrolled a high percentage of Medicare eligible men. Confounding from observational studies is a concern since observed differences in health status across and within racial or ethnic groups is likely due to a complex interaction of numerous factors, most of which are unmeasured and therefore impossible to control for statistically. For question 3 we included studies that examined the effect of provider characteristics on probability to be diagnosed and treated with different procedures. We also examined differences in outcomes after RP, the most common treatment for localized prostate cancer (and one in which volume was most likely to have an impact), in association with provider location, volume, and affiliations with academic centers. Eligible studies were administrative reports that measured outcomes in different locations, administrative surveys that measured physician distribution in regions of the United States, and epidemiologic studies that evaluated the association between provider characteristics and patient outcomes and had a control group. Inclusion criteria for the meta-analysis were as follows: studies reporting outcome rates by surgeon and hospital volume categories or relative risk of outcomes between groups with different surgeon and hospital volumes and studies with reported outcomes rates in different locations in the United States.
Table 2
Study inclusion criteria* for the key questions.
Articles were excluded if men with disease stage higher than clinical T1 or T2 were enrolled and outcomes were not stratified by stage. Studies were excluded if they were not published in English. We included nonrandomized studies of cryotherapy, IMRT, laparoscopic, or robotic prostatectomy, and HIFU that described men with T3/T4 disease because there is little known about outcomes associated with these treatments, commonly used for T1/T2 patients. Because of their recent introduction into clinical research and practice, these technologies are not addressed in the recent AUA clinical guideline. 29 For question 3 we excluded studies if the target population was outpatients or patients in long-term care facilities, there was no information regarding provider characteristics, or if there were administrative reports and single hospital studies with no control comparisons that did not test an associative hypothesis.
Types of participants. Men considered to have clinically localized prostate cancer (T1–T2, N0-X, M0-X) regardless of age, histologic grade or PSA level.
Types of interventions. For questions 1, 2, and 4 we included treatment options frequently utilized for men with clinically localized prostate cancer: RP (including laparoscopic or robotic assisted); WW, EBRT (including intensity modulated radiation, conformal radiation, photon beam), brachytherapy; ADT; HIFU, and cryotherapy.
From the AUA database, seven treatment categories, with 19 predefined treatments and the option of describing others that fit into each category, were identified. Four main categories were selected: prostatectomy (P), external beam radiotherapy, brachytherapy, and watchful waiting. Prostatectomy was broken down into radical prostatectomy and nerve-sparing prostatectomy (NSP); and EBRT was divided into EBRT and conformal EBRT. If a second treatment, such as hormone therapy, was used, that group was excluded. Data from randomized trials were also broadly categorized into these treatment options. Within category comparisons (e.g., different doses or methods of EBRT) are described in the broader categories. Emerging technologies were also evaluated based on discussion with TEP members, or internal content experts and feedback from peer reviewers (Appendix B). These included: IMRT, proton beam radiation, cryotherapy, HIFU, and laparoscopic or robot assisted RP.
Types of outcomes measures. The primary outcome for questions 1, 2 and 4 was overall survival. Additional outcomes include prostate cancer-specific survival, biochemical (PSA) metastatic and/or clinical progression free survival, health status, and quality of life. Adverse effects focused on common and severe AEs including bowel, bladder, and sexual dysfunction.
Assessment of the methodological quality of the studies. For question 3, assessment of study quality was based on the “Systems to Rate the Strength of Scientific Evidence scored from 0 (poorest) to 5 (highest). 30 Summated scores were used to establish study quality. The quality of evidence was estimated using U.S. Preventive Services Task Force criteria 31 and the AHRQ scale.
Data Extraction
Study, patient, tumor, and intervention characteristics as well as predefined outcomes were extracted by two researchers onto standardized forms. Standard errors, regression coefficients, and 95 percent CI were calculated from reported means, standard deviations, and sample size when provided/appropriate. 32 Decisions of study eligibility were made with no relation to authors and institutions. 33
Assessment of Risk of Bias
For Questions 1, 2, and 4 we assessed the risk of bias for the RCTs by evaluating several variables: 1) was there adequate allocation concealment during randomization, 2) were data analyzed based on the intention-to-treat-principle, and 3) did the trials have adequate length of followup and number of dropouts or lost to followup. The findings from RCTs were supplemented by the AUA Clinical Guideline Panel for Treatment of Clinically Localized Prostate Cancer database. This work is based on data extracted from 436 articles, primarily case series (over 80 percent), published between 1991 and April 2004. The potential for bias is considerable. The variability in reporting of results, lack of controls, and likelihood that the database contains results from multiple publications using identical or nearly identical populations limits data interpretation. For Question 3 we assessed the risk of bias by evaluating the adjustment for confounding patient and provider characteristics in observational studies. We conducted sensitivity analysis to estimate the differences in provider volume effect in studies with different adjustment strategies.
Applicability Assessment
Applicability of the population was estimated by evaluating the selection of the subjects in observational studies and clinical trials. Large observational cohorts based on the national registries and nationally representative administrative and clinical databases had high applicability. We conducted sensitivity analysis to examine the differences in provider volume effects in the studies that selected subjects from administrative and clinical databases and that reported random and convenience sampling of participants. Applicability of the intervention duration was high for the studies with followup more than 1 year and low for the studies with followup 6–12 months. No formal applicability assessment was conducted. To assess patients, treatments and outcomes most relevant to patients currently diagnosed with prostate cancer in the U.S. during the PSA era, we evaluated whether enrolled subjects were primarily detected by PSA testing. Health status and quality of life studies from nonrandomized studies were included if they were population based and in particular focused on Medicare eligible men. Based on knowledge from members of our Minnesota EPC, TEP members, and outside peer commentary, we focused on treatments most commonly used for early stage prostate cancer or emerging technologies. Outcomes of interest were selected based on similar feedback so as to be most relevant to clinicians and patients.
Data Synthesis
Due to differences in study designs, treatments tested, patient and tumor characteristics, and reporting of outcomes, we did not conduct pooled analysis for questions 1, 2, and 4. Summaries of effectiveness and AE outcomes with ranges according to treatment option, tumor characteristics, and group sample size are provided. Results are provided separately for randomized trials and nonrandomized studies.
For the AUA database, Minnesota EPC reviewers subsequently divided patients into groups for which the article provided data. For example, disease stage, PSA and Gleason scores, risk categories, and race were used to define groups. Within each group there were sometimes multiple subgroups. It was possible for subgroups to overlap. For the included graphs, each point represents an article/group combination. Some articles may have multiple points for any given time period or treatment. Due to the overlap between subgroups, the most inclusive groups available for each article were selected. When multiple subgroups overlapped, the total patients in the parent group along with subgroup definitions were used to select which subgroups would be used in the graphs. Gleason score was used for some of the graphs, so when a more inclusive definition was not available and Gleason score was used to define subgroups for an article, we tried to use those subgroups rather than subgroups defined by tumor characteristic or PSA, for example. Our primary goal was to assess the comparative effectiveness and adverse effects of the major treatment options for men with clinically localized prostate cancer overall and according to clinically relevant patient and tumor characteristics including: age (< vs. ≥65 years), race (White, Black, Hispanic, other), tumor stage (T1c [PSA detected] vs. other), PSA levels (≤4.0; 4.1–9.9; 10–19.9; ≥20.0 ng/ml), and Gleason histologic scores (2–4, 5–6, 7, and 8–10).
For question 3 the impact of the provider/hospital characteristics on clinical outcomes was estimated analyzing published evidence of the associations. Since no randomized trials were identified, observational studies were used to calculate the associations between outcomes and provider/hospital characteristics; both crude estimates and estimates adjusted for confounding factors. Relative risks of the outcomes among different providers/hospitals were analyzed.
The results of individual studies were summarized with relation to sample size and 95 percent CI. Weighted by the sample size (number of patients and hospitals) odds ratios and 95 percent CI were calculated with fixed and random effects models. 34 The results from random effects models are included in the report. The likelihood-based approach to general linear mixed models was used to analyze the association between independent variables and outcomes with the basic assumption that the data are linearly related to unobserved multivariate normal random variables. 35 Meta-regression models analyzed possible interactions with the year of data collection, databases to measure outcomes, and adjustment for confounding factors. 36, 37 The calculations were performed using STATA 38 and SAS 9.2 packages, Proc Mixed. 35
Consistency in the results was tested comparing the direction and strength of association in models with provider variables as continuous (overall trend) and categorical, in studies reporting outcome rates and adjusted relative risk, and with goodness of fit analysis. Chi squared tests were obtained to assess heterogeneity in study results. 36
Rating the Strength of the Body of Evidence
We rated the strength of the available evidence via a three-point scale (High, Medium, Low). High indicated consistent results from at least two high-quality studies with long-term followup. Medium included data from fewer then two high quality studies or studies that did not have long-term followup. Low confidence was from inconsistent results or studies of low quality or from populations with little relevance to current patients/practice.
- Methods - Comparative Effectiveness of Therapies for Clinically Localized Prosta...Methods - Comparative Effectiveness of Therapies for Clinically Localized Prostate Cancer
Your browsing activity is empty.
Activity recording is turned off.
See more...
