NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Nelson HD, Fu R, Humphrey L, et al. Comparative Effectiveness of Medications To Reduce Risk of Primary Breast Cancer in Women [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2009 Sep. (AHRQ Comparative Effectiveness Reviews, No. 17.)
This publication is provided for historical reference only and the information may be out of date.

Comparative Effectiveness of Medications To Reduce Risk of Primary Breast Cancer in Women [Internet].
Show detailsBackground
Breast cancer is the most frequently diagnosed noncutaneous cancer and the second leading cause of cancer death after lung cancer among women in the United States. In 2008, an estimated 182,460 cases of invasive breast cancer and 67,770 cases of in situ breast cancer were diagnosed, and 40,480 women died of breast cancer in the United States.
Recent clinical trials have demonstrated the efficacy of three medications—tamoxifen citrate, raloxifene, and tibolone—to reduce the risk of invasive breast cancer in women without pre-existing cancer. This therapy is sometimes referred to as “chemoprevention” in the literature, although this is not a fully accurate representation of the intervention. Tamoxifen and raloxifene are approved by the U.S. Food and Drug Administration for this indication and tibolone is not. Raloxifene is approved for use by postmenopausal women only. Current clinical recommendations, including those from the U.S. Preventive Services Task Force issued in 2002, support tamoxifen use for primary breast cancer prevention in women considered at high risk for breast cancer by the Gail model or other criteria and low risk for adverse events. However, use of risk-reducing medications for breast cancer is believed to be low in the United States.
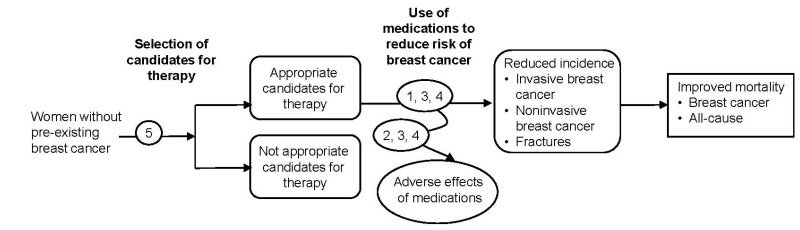
The purpose of this review is to evaluate the comparative effectiveness of tamoxifen citrate, raloxifene, and tibolone to reduce the risk of primary breast cancer; assess the nature and magnitude of harms; and examine how benefits and harms vary by age, breast cancer risk status, and other factors. The review was originally entitled “Comparative Effectiveness of Chemotherapy Agents in the Prevention of Primary Breast Cancer in Women.” Peer review comments suggested that the terms “chemotherapy” and “prevention” were misnomers. The term “medications to reduce risk” is a better representation of the intervention and therefore, all references to “chemoprevention” are edited, including the key questions and report title.
The review also examines issues related to clinical effectiveness, such as patient choice, concordance, adherence, and persistence of use, and evaluates methods to appropriately select patients for risk-reducing medications for clinical applications. The target population includes women without pre-existing breast cancer, noninvasive breast cancer, or precursor conditions who are not known carriers of breast cancer susceptibility mutations (BRCA1, BRCA2, or others). The analytic framework and key questions guiding this review are described below.
Key Question 1. In adult women without pre-existing breast cancer, what is the comparative effectiveness of selective estrogen receptor modulators (SERMs) tamoxifen citrate and raloxifene, and the selective tissue estrogenic activity regulator (STEAR) tibolone, when used to reduce risk for primary breast cancer on improving short-term and long-term outcomes including invasive breast cancer, noninvasive breast cancer, including ductal carcinoma in situ (DCIS), breast cancer mortality, all-cause mortality, and osteoporotic fractures?
Key Question 2. What is the evidence for harms of tamoxifen citrate, raloxifene, and tibolone when used to reduce risk for primary breast cancer?
Key Question 3. How do outcomes for tamoxifen citrate, raloxifene, and tibolone when used for primary prevention of breast cancer vary by heterogeneity in subpopulations?
Key Question 4. What is the evidence that harms or secondary potential benefits listed above affect treatment choice, concordance, adherence, and persistence to treatment with tamoxifen citrate, raloxifene, and tibolone when used for primary prevention of breast cancer?
Key Question 5. What methods, such as clinical risk-assessment models, have been used to identify women who could benefit from medications to reduce risk of breast cancer?
Conclusions
Key Question 1. Comparative effectiveness of tamoxifen citrate, raloxifene, and tibolone for the primary prevention of breast cancer, mortality, and fractures
- Eight large randomized controlled trials provide data on breast cancer risk reduction in women without pre-existing breast cancer. These include one good-quality head-to-head trial of tamoxifen and raloxifene and seven fair- and good-quality placebo-controlled trials (four tamoxifen, two raloxifene, and one tibolone). Results of placebo-controlled trials cannot be directly compared between types of medications because of important differences between study subjects.
- Tamoxifen (risk ratio [RR] 0.70; 0.59, 0.82; four trials), raloxifene (RR 0.44; 0.27, 0.71; two trials), and tibolone (RR 0.32; 0.13, 0.80; one trial) reduce the incidence of invasive breast cancer in midlife and older women by approximately 30 percent to 68 percent. Tamoxifen and raloxifene had similar effects in the STAR (Study of Raloxifene and Tamoxifen) head-to-head trial.
- Reduction of invasive breast cancer continued at least 3 to 5 years after discontinuation of tamoxifen in the two trials providing post-treatment followup data.
- Tamoxifen (RR 0.58; 0.42, 0.79; four trials) and raloxifene (RR 0.33; 0.18, 0.61; two trials) reduced estrogen receptor positive invasive breast cancer, but not estrogen receptor negative invasive breast cancer, in placebo-controlled trials. They had similar effects in the STAR head-to-head trial.
- Tamoxifen and raloxifene did not significantly reduce noninvasive breast cancer, including DCIS, in meta-analysis of four placebo-controlled trials, although noninvasive breast cancer was significantly reduced in the NSABP P-1 (National Surgical Adjuvant Breast and Bowel Project) tamoxifen trial (RR 0.63; 0.45, 0.89). The STAR head-to-head trial indicated no statistically significant differences between raloxifene and tamoxifen (RR 1.40; 0.98, 2.00).
- All-cause mortality is similar for women using raloxifene and those using tamoxifen, and also is similar for tamoxifen, raloxifene, or tibolone compared with placebo, although followup times in most trials were short. Tamoxifen does not reduce breast cancer mortality compared to placebo.
- Tamoxifen and raloxifene had similar effects on fractures at multiple sites in the STAR head-to-head trial. In placebo-controlled trials, raloxifene (RR 0.61; 0.54, 0.69; two trials) and tibolone (RR 0.55; 0.41. 0.74; one trial) reduced vertebral fractures; tamoxifen (RR 0.66; 0.45, 0.98; one trial) and tibolone (RR 0.74; 0.58, 0.93; one trial) reduced nonvertebral fractures; and tibolone reduced wrist (RR 0.54; 0.35, 0.82; one trial) but not hip fractures.
Table ASummary of primary prevention trials–benefits: number of events reduced with medications and strength of evidence
| Major health outcome | Head-to-head triala | Placebo-controlled trialsb | ||
|---|---|---|---|---|
| Raloxifene vs. tamoxifen | Tamoxifen vs. placebo | Raloxifene vs. placebo | Tibolone vs. placebo | |
| Invasive breast cancer | No difference | 7 (4, 12) +++ | 9 (4, 14) +++ | 10 (3, 17) ++ |
| Estrogen receptor positive | No difference | 8 (3, 13) +++ | 8 (4, 12) +++ | Insufficient |
| Estrogen receptor Negative | No difference | No difference ++ | No difference ++ | Insufficient |
| Noninvasive cancer | No difference | No difference + | No difference ++ | Insufficient |
| All-cause deathc | No difference | No difference +++ | No difference +++ | Insufficient |
| Vertebral fracture | No difference | No difference + | 7 (5, 9) +++ | 44 (25, 61) ++ |
| Nonvertebral fracture | Insufficient | 3 (0.2, 5) ++ | No difference +++ | 34 (8, 56) ++ |
- a
Study of Raloxifene and Tamoxifen (STAR).
- b
Number of events reduced compared to placebo per,1000 women-years assuming 5 years of use (95-percent confidence interval shown in parentheses).
- c
Based on short-term followup times from trials.
Strength of Evidence Symbols
- +++
High: Consistent results from numerous (>5) or large definitive trials show a positive protective effect.
- ++
Moderate: Some evidence (3–5 studies) suggests a protective effect, but results could be altered by future research.
- +
Low: Few (≤2) trials exist, existing trials have inconsistent results and/or limitations, results are likely to be altered by future research.
No difference Results are not statistically significantly different.
Insufficient Data are inadequate to calculate outcomes or are not reported.
Key Question 2. Harms of tamoxifen citrate, raloxifene, and tibolone when used for primary prevention of breast cancer
- In addition to the 8 large randomized controlled trials described in Key Question 1, harms data were provided by 12 placebo-controlled trials and 1 observational study of raloxifene, and 7 placebo-controlled trials and 1 observational study of tibolone.
- Raloxifene caused fewer thromboembolic events (RR 0.70; 0.54, 0.91) than tamoxifen in the STAR head-to-head trial. Tamoxifen (RR 1.93; 1.41, 2.64; four trials) and raloxifene (RR 1.60; 1.15, 2.23; two trials) cause more thromboembolic events than placebo. Risk returned to normal after discontinuation of tamoxifen in the two trials providing post-treatment data. Tibolone does not increase risk for thromboembolic events, although data are limited.
- Tamoxifen, raloxifene, and tibolone do not increase risk for coronary heart disease events, although data for tibolone are limited.
- Tibolone causes more strokes than placebo (RR 2.19; 1.14, 4.23); tamoxifen and raloxifene do not increase risk for stroke.
- In the STAR head-to-head trial, raloxifene caused fewer cases of endometrial hyperplasia (RR 0.16; 0.09, 0.29) and was associated with fewer hysterectomies (RR 0.44; 0.35, 0.56) than tamoxifen, but differences for endometrial cancer were not statistically significant (RR 0.62; 0.35, 1.08).
- Tamoxifen causes more cases of endometrial cancer than placebo (RR 2.13; 1.36, 3.32; three trials); raloxifene does not increase risk for endometrial cancer or uterine bleeding, and tibolone does not increase risk for endometrial cancer in clinical trials but was associated with more cases of endometrial cancer in a large cohort study (RR1.79; 1.43, 2.25).
- Raloxifene caused fewer cataracts (RR 0.79; 0.68, 0.92) and cataract surgeries (RR 0.82; 0.68, 0.99) than tamoxifen in the STAR head-to-head trial. Tamoxifen was associated with more cataract surgeries than placebo in the NSABP P-1 trial (RR 1.57; 1.16, 2.14). Raloxifene does not increase risk for cataracts or cataract surgery.
- In head-to-head comparisons, women using raloxifene reported more musculoskeletal problems, dyspareunia, and weight gain, while those using tamoxifen had more gynecological problems, vasomotor symptoms, leg cramps, and bladder control symptoms.
- Most common side effects for tamoxifen are hot flashes and other vasomotor symptoms, vaginal discharge, and other vaginal symptoms such as itching or dryness; for raloxifene, vasomotor symptoms and leg cramps; and for tibolone, vaginal bleeding and reduced number and severity of hot flashes.
Table BSummary of primary prevention trials–harms: number of events increased with medications and strength of evidence
| Major health outcome | Head-to-head triala | Placebo-controlled trialsb | ||
|---|---|---|---|---|
| Raloxifene vs. tamoxifen | Tamoxifen vs. placebo | Raloxifene vs. placebo | Tibolone vs. placebo | |
| Thromboembolic events | 6 (2, 10)c More with tamoxifen | 4 (2, 9) +++ | 7 (2, 15) +++ | No difference + |
| Coronary heart disease | No difference | No difference +++ | No difference +++ | No difference + |
| Stroke | No difference | No difference ++ | No difference ++ | 11 (1, 36) ++ |
| Endometrial cancer | No difference | 4 (1, 10) +++ | No difference ++ | Insufficient |
| Cataracts | 13 (5, 21) More with tamoxifen | No difference + | No difference +++ | Insufficient |
- a
Study of Raloxifene and Tamoxifen (STAR).
- b
Number of events increased compared to placebo per 1,000 women-years assuming 5 years of use (95-percent confidence interval).
- c
Number of events increased per 1,000 women-years assuming 5 years of use (95-percent confidence interval).
Strength of Evidence Symbols
- +++
High: Consistent results from numerous (>5) or large definitive trials show a harmful effect.
- ++
Moderate: Some evidence (3–5 studies) suggests a harmful effect, but results could be altered by future research.
- +
Low: Few (≤2) trials exist, existing trials have inconsistent results and/or limitations, results are likely to be altered by future research.
No difference Results are not statistically significantly different.
Insufficient Data are inadequate to calculate outcomes or are not reported.
Key Question 3. Variability of outcomes in subpopulations
- Tamoxifen and raloxifene had similar effects on breast cancer outcomes regardless of age and family history of breast cancer in the head-to-head STAR trial.
- Tamoxifen reduces breast cancer outcomes in subgroups evaluated in prevention trials based on age, menopausal status, estrogen use, family history of breast cancer, and history of lobular carcinoma in situ or atypical hyperplasia. In the NSABP P-1 trial, cancer rates were highest and risk reduction greatest among women in the highest modified Gail model risk category and among women with prior atypical hyperplasia.
- Raloxifene reduces breast cancer outcomes in subgroups evaluated in prevention trials based on age, age at menarche, parity, age at first live birth, and body mass index. Estimates from subgroups based on prior estrogen use, family history of breast cancer, and prior hysterectomy or oophorectomy are limited by smaller numbers of subjects.
- Thromboembolic events and endometrial cancer were more common in older (>50) than younger women in the NSABP P-1 trial.
- Tibolone causes more strokes in older (>70 years) than younger women.
Key Question 4. Treatment choice, concordance, adherence, and persistence to treatment with tamoxifen citrate, raloxifene, and tibolone when used for primary prevention of breast cancer
- Comparisons of adherence and persistence rates across medications in prevention trials are limited because few trials report treatment duration, completion rates, or other measures of adherence and persistence, and trials were designed for different treatment purposes.
- Discontinuation rates for tamoxifen or raloxifene are generally higher than placebo. In the few trials reporting discontinuation rates, the difference between treatment and placebo groups was ≤2 percent for adverse events and ≤4 percent for nonprotocol-specified events.
- Women make decisions to use tamoxifen for risk reduction based on their concern for adverse effects as well as their risk for breast cancer, according to small descriptive studies.
- Women weigh their physicians’ recommendations highly when deciding whether to take tamoxifen for risk reduction, according to descriptive studies of concordance.
- Studies of treatment choice and concordance for raloxifene and tibolone for breast cancer risk reduction are lacking.
Key Question 5. Clinical risk assessment models to identify women who could benefit from medications to reduce risk of breast cancer
- Nine risk stratification models that predict an individual’s risk for developing breast cancer have been evaluated for use in clinical settings. Models consider multiple risk factors for breast cancer.
- Risk stratification models demonstrate good calibration, with the expected number of breast cancer cases in a study population closely matching the number of breast cancer cases observed.
- All models have low discriminatory accuracy in predicting the probability of breast cancer in an individual. Most models perform only slightly better than age alone as a risk predictor.
- A Gail score of ≥1.66 percent has been used as a risk threshold in prevention trials and in Food and Drug Administration approval of tamoxifen and raloxifene for breast cancer prevention. However, this threshold has low discriminatory accuracy in predicting breast cancer in an individual.
Applicability
Trials met criteria for good applicability: they were conducted in settings appropriate to clinical practice, enrolled subjects selected with broad eligibility criteria, assessed health outcomes, and had followup periods of several years. Also, although inclusion criteria differed between trials, results for breast cancer outcomes were similar. For these reasons, the trials provided information about effectiveness as well as efficacy of the risk-reducing medications.
Clinicians can consider the results of trials to be most applicable to patients with characteristics similar to those of the study populations. Specifically, tamoxifen results apply to younger premenopausal and postmenopausal women meeting breast cancer risk criteria; tibolone results apply to older postmenopausal women with osteoporosis; and raloxifene results apply to postmenopausal women meeting breast cancer risk criteria and to older postmenopausal women with osteoporosis or cardiovascular disease and/or risk factors for cardiovascular disease. Women not well represented in the trials are those who are younger (<55 years old), have Gail scores <1.66 percent or considered low risk by other criteria used by some of the trials, are nonwhite, or are from outside North America and Europe. Also, premenopausal women were excluded from the raloxifene and tibolone trials.
Remaining Issues
While the efficacy of tamoxifen, raloxifene, and tibolone has been demonstrated for women in the clinical trials, it is not clear which women in clinical practice would optimally benefit from risk reduction. Future research to determine the optimal candidates for risk-reduction medications would help focus prevention efforts. Applying these findings to clinical selection criteria would improve identification of patients for risk-reducing medications in practice.
The results of current trials indicate that adverse effects differ between medications and may drive decisions for risk-reducing medications as much or more than benefits do. Further research to more clearly identify characteristics of individuals experiencing specific adverse effects would guide physicians and patients to regimens that cause the least harm.
- Executive Summary - Comparative Effectiveness of Medications To Reduce Risk of P...Executive Summary - Comparative Effectiveness of Medications To Reduce Risk of Primary Breast Cancer in Women
Your browsing activity is empty.
Activity recording is turned off.
See more...