NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Butler M, Olson A, Drekonja D, et al. Early Diagnosis, Prevention, and Treatment of Clostridium difficile: Update [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016 Mar. (Comparative Effectiveness Reviews, No. 172.)

Early Diagnosis, Prevention, and Treatment of Clostridium difficile: Update [Internet].
Show detailsLiterature Search Results
We identified 7416 unique citations (Figure 1) from 2010 to April 2, 2015. After excluding articles at title and abstract, full texts of 252 articles were reviewed to determine final inclusion. Six articles were added through hand search.
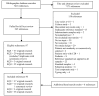
Figure 1
Literature flow diagram. CDI = C. difficile infection; KQ = Key Question
The appendixes of this report provide detailed information about the included studies: evidence tables (Appendix E); risk of bias and quality assessments of original research and systematic reviews (Appendix F); detailed analyses (Appendix G); and detailed strength of evidence assessments (Appendix H).
KQ1. How do different methods for detection of toxigenic C. difficile to assist with diagnosis of CDI compare in their sensitivity, specificity, and predictive values?
Thirty-seven new studies evaluated diagnostic tests for CDI. Twenty-three studies were from Europe, six from the United States, three from Korea, two from Canada, and one each from Australia, Mexico, and Saudi Arabia. Twenty-six studies were performed at a single center and 11 studies were multicenter studies. (See Appendix C for evidence tables.) Most studies included only unformed stool specimens. Overall, these studies, when combined with the 13 studies from the original review, include data on eight named immunoassays for Clostridium difficile toxins A and B, four GDH tests, 11 test algorithms, one LAMP, and 10 PCR. The number of studies assessing the diagnostic accuracy of tests that detect genetic material from Clostridium difficile in feces (LAMP and PCR) increased considerably—19 studies in the update compared to only three in the original review.
Table 3 provides a summary of the findings.
Table 3
Summary of diagnostic test findings new with the update.
The general rankings provided in Table 3 are based on the overall pattern of results summarized in Table 4, which shows the sensitivity, specificity, and negative and positive likelihood ratios (forest plots and ROCs in Appendix G) comparisons (which are not derived from direct comparisons between test classes). In short:
Table 4
Summary of pooled diagnostic tests by test class.
- A negative LAMP assay is as effective at decreasing the probability that a patient has CDI as PCR and GDH assays and is more effective than Toxin A/B and algorithmic approaches. A positive LAMP assay is likely more effective at increasing the probability that a patient has CDI than PCR, Toxin A and/or B tests, and GDH assays but is less effective than algorithmic approaches.
- A negative PCR test is as effective at decreasing the probability that a patient has CDI as LAMP and GDH assays and more effective than Toxin A/B and algorithmic approaches. A positive PCR for CDI is more effective at increasing the post-test probability that a patient has CDI than a positive GDH test, similarly effective to LAMP and Toxin A/B assays, and less effective than algorithmic approaches.
- A negative immunoassay for Toxin A and/or B is as effective as algorithmic approaches but is less effective than PCR, LAMP, and GDH tests at decreasing the likelihood that a patient has CDI. A positive immunoassay for Toxin A and/or B is more effective at increasing the post-test probability that a patient has CDI than a positive GDH test, similarly effective to PCR, and less effective at increasing the probability that a patient has CDI than LAMP and algorithmic approaches.
- A negative GDH assay is as effective at decreasing the probability of CDI (albeit with less precision in the estimate) as PCR and LAMP and more effective than Toxin A and/or B tests and algorithmic approaches, but a positive GDH assay is less effective at increasing the probability that a patient has CDI than all the other test classes.
- A negative algorithmic test for CDI is the one of the least effective tests at decreasing the probability that a patient has CDI, while a positive test for CDI via an algorithmic test is the most effective approach to increase the post-test probability that a patient has CDI.
Heterogeneity within the classes is not easily explained by test type alone. The reasons for the differences in the operating characteristics between individual tests within the same class and between classes of tests are not well described in the studies, and while studies were selected to have good internal validity, studies may have differed significantly in the conduct of the tests and the patient populations.
We found no studies that met the inclusion criteria that provided sufficient sample characteristics to evaluate whether performance measures varied systematically based on health system, laboratory, training methods, or patient characteristics. Similarly, no studies that met the inclusion criteria evaluated the effect of different assays for CDI on health systems or patient outcomes.
KQ2. What are effective prevention strategies?
Fourteen new articles examined prevention studies; one systematic review (which included five new studies), two on chlorhexidine gluconate bathing of patients, two on using hydrogen peroxide vapor for room disinfection, two on hand hygiene, one on disposable hydrogen peroxide wipes, one on gloving, and four on multicomponent interventions. None were controlled trials. Two used quasi-experimental designs, four (plus all relevant studies in the systematic review) used interrupted time series analysis, four used prospective pre/post designs of single sites, and one used a retrospective pre/post designs for a single site. No study reported that it was conducted in an outbreak setting.
Overall, while study design and reporting improved somewhat from the original review, the evidence available to link prevention strategies to clinically important outcomes, such as CDI incidence, remains low strength. Table 5 provides a summary of the findings. (See Appendix G for evidence table.)
Table 5
Summary of prevention findings new with the update.
Antibiotic Stewardship
One new high-quality systematic review34 of antibiotic stewardship practices in inpatient settings included six studies (one RCT and five interrupted time series) and overlapped with the original review by one interrupted time series study.35 The new systematic review categorized stewardship practices into audit and feedback, formulary restrictions and preauthorization interventions, guidelines implemented with feedback, guidelines without feedback, and computerized decision support programs. The six studies were evaluated as providing low strength of evidence that antibiotic stewardship programs reduced CDI incidence within the four antibiotic use program categories examined (audit and feedback, formulary restrictions, guidelines with feedback, and computerized decision support).34 The review found no reports of harms associated with stewardship programs.
Transmission Interruption
Bathing patients was a new form of transmission interruption from the updated literature. Two moderate risk of bias studies found chlorhexidine gluconate bathing had inconsistent findings. One cluster randomized crossover study found no effect for daily bathing in five ICUs in one medical center with either intention-to-treat or as-treated analysis.36 This study did not assess compliance and experienced relatively low CDI rates in both study arms. In contrast, one quasi-experimental study found that bathing either 3 days per week or daily in three cohorts within one hospital reduced CDI rates.37 While there were no concurrent controls, the cohort design allowed for some replicability and comparison. Changes in effects with dosing (daily versus three times per week) and the wash-out period strengthen the findings. Highest compliance rates were found in the ICU cohort versus general hospital or medical/surgical cohorts; however, regression models did not find compliance associated with CDI rates; the model estimated RR 0.71 (CI 0.57 – 0.89) three times per week for all cohorts.
Two new studies examined terminal room cleaning including hydrogen peroxide vapor.38,39 Rooms known to have prior occupants with CDI or other disease-causing organisms are sealed and sporicidal hydrogen peroxide vapor is released into the rooms in a gassing process. Protocols are followed for sealing the vapors within the rooms until proper ventilation is complete. One pre/post study in the original review used hydrogen peroxide vapor as part of a multicomponent intervention to respond to an abrupt increase in nosocomial CDI infections (Table 4 in the original report). The decrease in CDI infections could not be separated from the natural decline that follows epidemics, nor could the effect of the vapor be separated from the multiple component intervention. Both new studies occurred in large (900-bed) hospitals not facing epidemic or hyperendemic events. One pre/post study of the vapor versus standard cleaning with bleach found a statistically significant reduction in CDI incidence.38 In contrast, the quasi-experimental cohort study used hydrogen peroxide liquid in the standard cleaning solution and found a trend in reduction but no statistical difference in CDI.39 Of the three studies, cleaning time ranged from 2 hours 20 minutes to 3 – 4 hours per room.
Hydrogen peroxide disposable wipes for daily cleaning of high-touch surfaces were used in one interrupted time series study.40 The study reported CDI rates dropped from 54 to 39 cases/10,000 patients (p=.0005) when compliance was >80 percent. CDI rates were not different for any cleaning compliance level. The study did not report results compared to a control hospital, however, the control did not monitor compliance, and the patient population was younger.
One new pre/post study examined the effect of portable pulsed xenon ultraviolet light after terminal room cleaning on CDI incidence in a single 140-bed community hospital.41 Rooms with a previous CDI patient were cleaned with a chlorine-based disinfectant product, followed by one 7-minute exposure in the bathroom and two 7-minute exposures in the main room to the ultraviolet light. The lights were also used in the operating suites at night, emergency departments in the early mornings, and other clinical areas as available. CDI incidence and hospital-associated CDI deaths and colectomies were found to decline.
Two new studies examined the effect of handwashing campaigns on CDI rates using uncontrolled interrupted time series design in 187 hospital trusts in the England42 and 166 acute care hospitals in Ontario, Canada.43 The original report did not locate studies that directly addressed the effect of handwashing on CDI. The two new studies examined campaigns that incorporated education and training programs and monitoring and feedback through either internal reports42 or public reporting.43 The program in England also empowered patients to remind healthcare workers of hand hygiene.43 Based on the one moderate risk of bias study from England, low-strength evidence suggests that handwashing campaigns can reduce CDI incidence over a 3-year period, with rates falling from 16.75 to 9.49 cases per 10,000 bed days. The study also found via regression model that soap use (measured via centralized procurement of soap) was independently associated with a slight reduction in CDI.42 The other high risk of bias study found no statistical difference; however, the authors note having been unable to adjust for several possible confounders, including patient location in the hospital, type of hand product used, and concomitant introduction of other hospital-level infection prevention and control interventions.
One new pre/post study examined universal gloving with emollient-impregnated gloves.44 This study does not add significantly to the original report's finding of low-strength evidence for gloving based on one RCT (Table 4 in the original report).
Cleaning and disinfection studies reported no adverse events noted for chlorhexidine gluconate bathing37 or hydrogen peroxide vapor.38,39 Changes in mortality associated with a stewardship can be considered a harm, if the difference in mortality is due to changes in prescribed antibiotics. The systematic review noted mortality as a primary outcome; of the six studies reporting CDI incidence, four reported mortality outcomes with no significant differences between comparisons. Otherwise, harms were not reported for antimicrobial stewardship programs.
Multiple Component Studies
Five new high risk of bias studies used multiple component interventions to address reducing CDI rates.45-49 Three used pre/post designs45,46,49 and two used uncontrolled interrupted time series approaches (both at single hospitals).47,48 Ten studies with pre/post or time series with pre/post statistical approaches were identified for the original review (Table 4 in the original report).
Some differences in the literature from the original review are noted. First, the studies were framed as responding to general heightened concerns for CDI as a hospital associated pathogen rather than a localized epidemic or high endemic. Second, study followup was longer, ranging from 2 – 3 years for pre/post studies45,46 to 27 – 81 months for time series studies.47,48 Third, studies tended to include more information on CDI definitions and laboratory testing methods.
The study designs do not permit inferences for individual intervention components; however, the increase in study periods suggests that multiple component interventions can be sustained over several years.
KQ3. What are the comparative effectiveness and harms of different antibiotic treatments?
Three studies met inclusion criteria: an RCT comparing fidaxomicin to vancomycin,50 a three-arm RCT comparing tolevamer (a toxin-binding resin) to metronidazole and vancomycin,51 and a three-arm prospective cohort study comparing intravenous metronidazole to oral metronidazole and vancomycin.52 Data from these new studies were combined with studies from the original report—a previous RCT of fidaxomicin versus vancomycin, and with three previous RCTs comparing metronidazole and vancomycin—to assess the efficacy of each drug.
Table 6 provides a summary of the findings. (See Appendix C for evidence tables.)
Table 6
Summary of standard treatment findings using pooled RCT data from original report and update.
Benefits
The findings that vancomycin is more effective for initial cure of CDI in adults is new to this update because of improved precision. While the results for fidaxomicin versus vancomycin are consistent with the original review, the strength of the evidence improved.
An observational study (n = 205) comparing oral metronidazole, intravenous metronidazole, and vancomycin was also identified.52 Results are similar to the RCTs, so this study was not included in the analyzed set. Initial cure was comparable for oral vancomycin (81 percent) and oral metronidazole (82.6 percent), but was significantly lower for intravenous metronidazole (52.4 percent; P <.001). Intravenous metronidazole performed significantly worse than either oral drug.
Time to resolution of diarrhea was reported in both the newly identified RCTs, with no differences observed based on treatment received. This outcome was not reported in the observational study. For both time to resolution of diarrhea and mortality, results did not differ from the original review's finding of no differences.
Harms
Only a slight change was observed based on the newly included studies. Similar to the original report, in the trial of metronidazole versus vancomycin, a similar percentage of subjects in each treatment arm experienced one or more serious adverse events. However, more subjects in the metronidazole group discontinued study medication because of an adverse event (11.2 percent versus. 6.5 percent; P = .06), whereas more subjects in the vancomycin group had evidence of nephrotoxicity (4.6 percent versus 1.0 percent, P = .02). Other harms, such as antimicrobial resistance, were not reported.
Disease Severity
In both new RCTs, pre-specified subgroup analyses among subjects with severe disease were performed to assess differences in outcome by treatment arm. Disease severity was generally determined by one or more clinical values such as white blood cell counts, serum creatinine concentrations, body temperature, and severity of abdominal pain due to CDI. No significant differences were observed for initial cure for severity subgroups. Analyzing by disease severity did not change the overall study results. One study found less recurrence for vancomycin versus metronidazole for severe disease, but the results varied based on whether per-protocol, modified intention to treat, or strict intention to treat analyses were used. The observational study also looked for a treatment effect when stratified by disease severity and found no significant differences. The original review found insufficient evidence for treatment by severity based on one post hoc subgroup analysis for vancomycin versus metronidazole.
KQ4. What are the effectiveness and harms of other interventions?
Other treatments were categorized as (FMT, probiotics, or other. FMT was the largest updated literature set for nonantibiotic adjunctive therapy. Twenty-three new studies examined FMT for CDI: three RCTs and 20 observational studies, in addition to three observational studies carried forward from the original review. We identified 12 new studies on probiotic use: 10 RCTs and two observational studies, in addition to seven RCTs included in the prior report. We identified three new RCTs on other nonstandard therapies.
Table 7 summarizes the findings. (See Appendix C for evidence tables.)
Table 7
Summary of nonstandard treatment findings using data from original report and update.
FMT for Recurrent CDI
Twenty-three new studies addressed FMT for recurrent CDI; three were small size RCTs and the others were case series. Most studies were small, enrolling 12 to 94 individuals. Followup was variable, and ranged from 3 weeks to 8 years. In most cases, FMT was described as being administered after antimicrobials had reduced or resolved the acute symptoms of CDI, with the goal of limiting subsequent recurrence.
The three RCTs are noteworthy. One unblinded, three-arm RCT, conducted in the Netherlands, enrolled 43 adults with recurrent CDI (mean age 70, 43 percent women).53 Patients were randomized to oral vancomycin, FMT, or vancomycin plus bowel lavage. Followup was 10 weeks and the endpoint was resolution of diarrhea. The study was stopped early due to a large difference between the FMT and comparator groups (81 percent versus 31 percent and 23 percent), largely due to an unexpectedly low response rate in the group randomized to vancomycin. FMT was administered via nasoduodenal tube. The resolution of diarrhea rate in the two vancomycin arms was considerably lower than the anticipated 60 percent. This may have been due to chance, and the 60-percent rate may have been achieved had the study treated the expected 38 patients per arm. However, without having run the full course, the study effect size remains uncertain.
Cammarota and colleagues conducted an additional unblinded trial of FMT via colonoscopy versus a vancomycin regimen that was given for at least 3 weeks, with the latter half given in a pulsed fashion (dosed every 2-3 days).54 In patients with pseudomembranous colitis, the FMT protocol was amended after two patients to give FMT infusions every 3 days until resolution of colitis, versus the single infusion given to patients with CDI without pseudomembranous colitis. This study enrolled 39 subjects, with a mean age of 73. The primary endpoint was resolution of diarrhea associated with CDI at 10 weeks after the end of treatment. When analyzed by resolution after a single course of treatment (FMT or vancomycin), 65 percent of subjects had resolution of diarrhea with FMT, versus 26 percent with vancomycin. The authors noted that administering multiple courses of FMT increased the success rate to 90 percent in the FMT group, and that multiple antibiotic courses increased the success rate to 53 percent in the vancomycin group. This study was also stopped early after an interim analysis.
Youngster and colleagues conducted an unblinded RCT that randomized 20 individuals with recurrent CDI (mean age 54) to colonoscopic or nasogastric administration of FMT.55 The study endpoint was resolution of diarrhea without relapse within 8 weeks. The authors found no difference between the two modalities of FMT administration, with an overall success rate of 70 percent after one treatment.
Based on a qualitative analysis of the unpooled data (Appendix G), low-strength evidence showed that FMT resolves diarrhea and prevents relapse in people with recurrent CDI.
FMT for Refractory CDI
Three studies reported outcomes for FMT in individuals with refractory CDI (defined as an episode that did not respond to antibiotic treatment; clearly identified by study authors). All were from case series, totaling 19 individuals.56-58 Overall, there was insufficient strength of evidence supporting the role of FMT in refractory CDI. Unfortunately, few FMT studies provided detailed patient information to identify whether included patients could be considered refractory. For instance, in one study of 94 patients receiving FMT for recurrent or refractory CDI, there was not a detailed accounting of how many had refractory versus recurrent disease.59
Probiotics for CDI
Nineteen studies reported use of probiotics as adjunctive treatment for CDI: Ten RCTs and two observational studies were newly identified, while seven RCTs were included in the prior report. With 17 RCTs to provide a best evidence base, the observational studies will not be discussed further.
In all studies, probiotics were administered as an adjunct to standard antibiotic treatment to prevent CDI. All studies included adult inpatients or outpatients with a mean reported age of 50 to 77 years. The studies enrolled 40 to 2981 subjects. The probiotics tested were lactobacilli species in six studies, saccharomyces species (S. boulardii) in six studies, and multiorganism in five studies: both lactobacillus and saccharomyces species in one study, lactobacillus and bifidobacterium in two studies, a four-strain preparation of three lactobacilli and bifidobacterium in one study, and VSL#3 in one study.
For quantitative analysis, we categorized probiotics as single organism (lactobacillus organisms only), S.boulardii, or multiorganism (e.g., multistrain preparation of lactobacilli and bifidobacteria). Overall, we found low-strength evidence that probiotics containing only lactobacillus organisms are more effective than placebo in preventing an acute episode of CDI, predominantly driven by one moderate risk of bias study that also demonstrated dose response. We found low-strength evidence that probiotics containing S.boulardii given as adjunct to standard antimicrobial therapy are comparable to placebo in preventing an episode of CDI. We also found low-strength evidence that the multiorganisms are more effective than placebo.
Other Treatment Agents for CDI
Rifaximin versus placebo after standard antibiotic for CDI was examined by Garey and colleagues.60 Rifaximin is a nonabsorbable antibiotic with FDA approval to treat traveler's diarrhea. Sixty-eight individuals with CDI (mean age 61, 50 percent were women) were treated for 20 days. After 3 months of followup, authors reported no statistically significant difference in recurrent CDI between groups. Recurrent diarrhea was reported less likely in the rifaximin group, but this included self-reported diarrhea episodes without confirmed C. difficile toxins.
Human recombinant lactoferrin versus placebo was examined by Laffan and colleagues.61 Human recombinant lactoferrin from breast milk has both anti-inflammatory and antimicrobial properties. The study randomized 30 residents of a long-term care facility beginning a new course of antibiotic, either with CDI or without, to human recombinant lactoferrin or placebo for 8 weeks. Mean age was 62, 64 percent were women, and 32 percent were black. The study endpoint was CDI incidence rates at days 14, 42, and 56. CDI rate did not differ statistically between groups.
Cholestyramine, a toxin-binding substance, was used to prevent CDI in one case series of 46 Lyme disease inpatients receiving ceftriaxone.62 Three patients subsequently developed CDI. Patients received 4 g/day administered orally up to 1 hour after the intravenous ceftriaxone but more than 1 hour before the evening meal. Patients were followed 30 days after treatment end. The lab-confirmed CDI rate of 6.5 percent (3/46 patients) was lower than reported rates for other patients receiving cefrtriaxone.
Harms of Adjunctive Treatments
Harms for FMT were available in the updated literature set. Adverse events after FMT in the single small RCT were diarrhea, cramps, belching and nausea, and constipation.53 Serious adverse events included one hospitalization and two cases of infections, unrelated to FMT. Risk of infection from FMT appears to be low, but is also dependent on the donor screening and testing process, especially for pathogens without widely available diagnostic tests, such as norovirus or rotavirus. Upper gastrointestinal bleeding was reported in one study with nasogastric administration of FMT.63 Other serious adverse events were peritonitis, pneumonia, and microperforation of the colon.64 All-cause mortality after FMT ranged from 0 – 25 percent when reported, depending on the length of followup. Mortality rates after FMT were higher in individuals with refractory compared with recurrent CDI. However, variable followup time, differences in baseline comorbidities, and especially the lack of any control group make placing this figure into context difficult. Whether deaths were due to FMT or reflected the overall poor health status of individuals undergoing FMT was unclear, particularly for those with refractory CDI. While one study reported a followup interval of up to 8 years,65 the followup for the majority of studies was 3 months or less. Therefore, the long-term (greater than 3 months) adverse effects of FMT are largely unknown.
Sixteen of 19 studies of probiotics as adjunctive treatment for CDI reported data on adverse events (see Appendix Table E5). Treatment with probiotics was not associated with increased risk of adverse events in any of the studies. No serious adverse events were reported that were attributed to probiotic treatment, although followup was typically 4 weeks or less, with two RCTs extending followup to 12 weeks. Given the importance of the potential harm due to probiotics, we reiterate from the original report that fungemia may be a serious potential harm associated with administration of probiotics for CDI in critically ill patients.66
- Literature Search Results
- How do different methods for detection of toxigenic C. difficile to assist with diagnosis of CDI compare in their sensitivity, specificity, and predictive values?
- What are effective prevention strategies?
- What are the comparative effectiveness and harms of different antibiotic treatments?
- What are the effectiveness and harms of other interventions?
- Results - Early Diagnosis, Prevention, and Treatment of Clostridium difficile: U...Results - Early Diagnosis, Prevention, and Treatment of Clostridium difficile: Update
Your browsing activity is empty.
Activity recording is turned off.
See more...