NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Ervin AM, Boland MV, Myrowitz EH, et al. Screening for Glaucoma: Comparative Effectiveness [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012 Apr. (Comparative Effectiveness Reviews, No. 59.)
This publication is provided for historical reference only and the information may be out of date.
Background
Glaucoma is a leading cause of visual impairment and blindness and affects approximately 60.5 million people worldwide.1,2 Although glaucoma may be characterized by optic nerve damage, visual field loss, and elevated intraocular pressure, there is no consensus definition for confirming diagnosis.3 Damage is irreversible, so early detection can prevent severe vision loss. Open-angle glaucoma (OAG), the most common subtype of the disease, affects more than 2.5 million people in the United States, with a median age-adjusted prevalence of 4.6 percent among black people and 1.6 percent among white people (based on year 2000 estimates).4
Unfortunately, it has been shown that only half of the prevalent cases of glaucoma have been identified in the United States due to at least two factors.4 First, glaucoma is an asymptomatic disease that patients do not notice until the onset of advanced disease, accompanied by severe vision loss. Second, there is no single test to identify people with glaucoma, which has severely hampered the establishment of screening-based programs to detect the disease.
The March 2005 U.S. Preventive Services Task Force (USPSTF) recommendation addressing screening for glaucoma stated that there was “insufficient evidence to recommend for or against screening adults for glaucoma.” The USPSTF noted that intraocular pressure measurement and optic nerve head assessment alone have limited effectiveness as population-based screening tools.5,6 The USPSTF also concluded that methods used to assess visual field loss may be impractical for population-based screening due to the length of time required for testing and the challenge of equipment portability. Since 2005, there have been significant advances in the devices used to assess optic nerve structure and function,5,6 with several published studies on new diagnostic tests, such as frequency doubling technology, used to assess visual field loss. Because of this new evidence, we believe that a re-evaluation of the safety and effectiveness of population-based glaucoma screening is warranted.
Objectives
The objective of this review was to summarize the evidence regarding the safety and effectiveness of screening-based programs for OAG, with a specific focus on the effects of screening on visual impairment, patient-reported outcomes, intraocular pressure, visual field loss, optic nerve damage, and adverse effects. The effect of screening on these outcomes is considered in the context of treatment of those who, after having been screened, are diagnosed as having glaucoma. This review also includes a summary of the diagnostic accuracy of screening examinations and tests for OAG.
Key Questions (KQs)
KQ1
KQ1a: Does a screening-based program for open-angle glaucoma lead to less visual impairment when compared with no screening program?
KQ1b: How does visual impairment vary when comparing different screening-based programs for open-angle glaucoma?
KQ2
KQ2a: Does a screening-based program for open-angle glaucoma lead to improvements in patient-reported outcomes when compared to no screening?
KQ2b: How do patient-reported outcomes vary when comparing different screening-based programs for open-angle glaucoma?
KQ3
What is the predictive value of screening tests for open-angle glaucoma?
KQ4
KQ4a: Does a screening-based program for open-angle glaucoma lead to reductions in intraocular pressure when compared with no screening program?
KQ4b: How does intraocular pressure vary when comparing different screening-based programs for open-angle glaucoma?
KQ5
KQ5a: Does a screening-based program lead to a slowing of the progression of optic nerve damage and visual field loss when compared with no screening program?
KQ5b: How do optic nerve damage and visual field loss vary when comparing different screening-based programs for open-angle glaucoma?
KQ6
What are the harms associated with screening for open-angle glaucoma?
Analytic Framework
The analytic framework (Figure A) depicts the impact of both screening and treatment for OAG. It depicts the KQs within the context of the inclusion criteria described in the following sections. The figure depicts how screening-based (S) programs, which may incorporate treatment when indicated, may reduce visual impairment (S: KQ1) and/or improve patient-reported outcomes (S: KQ2), reduce intraocular pressure (S: KQ4), and possibly slow the progression of optic nerve damage and/or visual field loss (S: KQ5). The figure also incorporates the potential predictive value of screening-based programs to detect OAG and people suspected of having OAG (S: KQ3). Finally, the potential for harms of screening (S: KQ6) are illustrated in the framework.
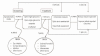
Figure A
Analytic framework for screening and treatment for open-angle glaucoma. KQ = Key Question; T = Key questions for the Comparative Effectiveness of Treatment for Glaucoma; S = Key questions for the Comparative Effectiveness of Screening for Glaucoma
Methods
Input From Stakeholders
The Agency for Healthcare Research and Quality (AHRQ) requested that the Johns Hopkins University Evidence-based Practice Center (JHU EPC) assist with the formulation and refinement of the Comparative Effectiveness Review (CER) topic, effectiveness of screening and treatment for glaucoma. In consultation with AHRQ, the JHU EPC investigators identified a small group of stakeholders to serve as members of a Key Informant Group. The Key Informant Group helped shape the KQs relevant to the topic by providing input regarding the populations and clinical subgroups; interventions; and outcomes of interest to clinicians, policymakers, payers, and consumers.
The EPC investigators incorporated the feedback of the Key Informants into a draft of the KQs, analytic framework, and inclusion criteria. A draft of the KQs was posted on the AHRQ Web site for public comment from April 22 to May 20, 2010. The investigators finalized the inclusion criteria after considering the public comments.
A Technical Expert Panel (TEP) was selected to provide broad expertise and perspectives specific to the topic. The TEP reviewed the proposed methodological approach for completing the CER and provided information to the EPC to aid in the refinement of the inclusion criteria and literature search strategies. The final protocol, titled The Comparative Effectiveness of Screening for Open-Angle Glaucoma, was posted to the AHRQ Web site on November 16, 2010.
Data Sources and Selection
We included randomized controlled trials (RCTs), quasi-randomized controlled trials, and observational study designs, including cohort and case control studies, for KQs 1 through 6. For KQ3 we also included cross-sectional studies, study designs in which all tests (including the index, comparator, and reference standard) were performed on all participants, and designs in which participants were randomized to one test (among the index and potential comparator(s)) but all were evaluated with the reference standard.7 We excluded case series of fewer than 100 participants, as studies smaller than this are expected to identify events occurring at a rate of less than 3 percent. We excluded conference abstracts that met our study inclusion criteria, as we did not have the resources to contact the study investigators with additional queries before the conclusion of data abstraction. We included systematic reviews that addressed the KQs. We excluded studies that addressed the following:
- Prevalence of glaucoma in a specific population, unless the studies also included tests of diagnostic accuracy
- Disease progression that did not include participants previously screened for glaucoma
- Risk factors for glaucoma
Types of Participants
We included studies of adult (as defined by included studies) asymptomatic participants in general or high-risk populations. For both populations we excluded studies of participants previously tested, diagnosed with glaucoma, or presenting with symptoms known to be related to a diagnosis of glaucoma. Asymptomatic high-risk populations included those with a family history of glaucoma; those from specific racial/ethnic groups; those with specific ocular or other medical conditions, as defined by included studies (e.g., diabetes); and older age groups, as defined by included studies.
We also included studies of suspected OAG subpopulations, which included participants identified from prior testing as possibly having glaucoma or as having a risk factor for glaucoma (e.g., high intraocular pressure), but with an unconfirmed diagnosis. We excluded studies of participants with known glaucoma at the time of screening (KQs 1, 2, 4, and 5) and those that included the healthy eye of a participant with known glaucoma (KQ3). We excluded studies in which the candidate tests were performed on a sample of healthy volunteers only. We did not exclude studies that enrolled healthy volunteers in addition to those with suspected glaucoma at the time of screening.
Interventions
We included studies of the following screening tests conducted alone or in any possible combination (including multicomponent simultaneous or sequential testing):
- Direct and indirect ophthalmoscopy
- Fundus photography or computerized imaging of the posterior pole, optic disc, or retinal nerve (optical coherence tomography (OCT; with the exception of OCT 1 and OCT 2), retinal tomography, scanning laser polarimetry)
- Pachymetry (corneal thickness measurement) when used in conjunction with another test to diagnose glaucoma; we excluded studies where pachymetry was used alone
- Perimetry (including short-wavelength, high-pass, motion, flicker perimetry, yellow and blue perimetry)
- Tonometry (contact and noncontact tonometry)
We excluded studies of the following screening tests and related analysis software that are either (1) not commercially available for screening or (2) not commonly used or no longer used in the diagnosis of glaucoma:
- Contrast sensitivity and visual acuity
- Electroretinography
- Heidelberg retina tomograph (HRT) I (confocal scanning laser ophthalmoscope)
- Optical coherence tomography (OCT) 1 and OCT 2
- Tests of color vision
- Versions of the GDx (scanning laser polarimeter) without corneal compensation
- Water drinking tests
We also excluded studies that examined only technical aspects of included devices (e.g., usability, technician training).
Screening and Diagnostic Device Descriptions
Below are detailed descriptions of the devices and tests included in this CER, with information on mechanism, operation, and skill required to complete and interpret each test.
Tests of Optic Nerve Structure
Heidelberg Retinal Tomography
The Heidelberg retina tomograph is a scanning laser ophthalmoscope that can create three-dimensional images of the retina and optic nerve head. After the images are collected, the device analyzes them to calculate values such as the area of the optic nerve head, the area and volume of the neuroretinal rim, the ratio of the area of the optic nerve head “cup” to the disc, and many others. The current versions of the device also compare values obtained for a particular patient with those of a population of healthy persons to estimate the probability of optic nerve disease consistent with glaucoma. Reports of these data can then be used by clinicians to diagnose either new or progressive disease.
The device itself consists of a table-mounted unit with imaging optics and a connected computer to allow for image acquisition and management of patient data. As such, the system is not easily portable. Operation of the device also requires personnel who have been trained to operate the software and hardware. This training includes not only the basics of entering patient information but also trouble-shooting problems with image quality and patient positioning.
Optical Coherence Tomography
An optical interferometer is used to create cross-sectional images of ocular structures, including the retina and optic nerve head. Once the images are collected, they can be analyzed and various anatomic layers can be segmented for further analysis. Such analysis of the retinal nerve fiber layer and structure of the optic nerve head is relevant to the diagnosis of glaucoma.
The original OCT devices all used time-domain analysis of the collected data. Thus, the time to collect an image was a significant limitation to the resolution that could be achieved. More recently, spectral-domain devices have become available; they can collect higher resolution images in the same time required to collect lower resolution images using the time-domain devices.
As with the HRT, the OCT machines all consist of a table-mounted unit with the optics connected to a computer for image acquisition and analysis. There are more portable versions of the optics available, but they still require a connection to computational power for image analysis. OCT devices also require trained personnel to operate them effectively.
Optic Disc Photography
After hand drawing, photographs are perhaps the earliest method of documenting the appearance of the optic nerve head. Photographs can be taken as single images, nonsimultaneous stereo pairs in which the camera is moved slightly between images, and simultaneous stereo pairs in which two images are captured at the same time. The advantage of stereo photographs is that they enhance the reviewer’s ability to assess optic nerve structures. Although optic disc photographs were first captured on film, they now are captured using digital technology. Historically, obtaining good-quality photographs required a trained ophthalmic photographer and an expensive camera system. As the systems have become more computerized and the optics more refined, the skill required to acquire adequate images has declined to the point where some telemedicine systems no longer require specially trained operators.
The analysis of optic nerve photographs is currently less quantitative than analysis for the imaging techniques previously discussed. Although computerized analysis of digital images is improving, good-quality evaluation of disc photographs requires significant skill on the part of the examiner.
Retinal Nerve Fiber Layer (RNFL) Photography
RNFL photography is a specialized photographic technique using red-free (green) light to image the RNFL. Green light is absorbed by the melanin in the retinal nerve fiber, and the striations become visible as they radiate around the optic nerve. RNFL photographs permit comparisons over time and can help detect diffuse or localized RNFL loss consistent with glaucoma. RNFL photographs are difficult and often uncomfortable for the patient, and require specialized equipment and trained photographers. For these reasons and because they are difficult for clinicians to interpret, they rarely are used in clinical practice.
Scanning Laser Polarimetry (SLP)
The scanning laser polarimeter assesses the RNFL using polarized light to measure the phase shift that occurs due to the presence of repetitive microstructures. The size of the shift depends on both the thickness and integrity of the RNFL. The cornea also contains repeating structures that affect polarized light, so the commercial version of the scanning laser polarimeter has undergone multiple revisions to accommodate this effect. The images collected by SLP can be analyzed to assess the thickness of the RNFL, which is directly related to glaucomatous damage.
The company that manufactures the commercially available SLP (GDx, Carl Zeiss Meditec) has designed the device as a single table-top unit that does not require a separate computer, unlike the OCT and HRT. As with other available devices, however, training is required to obtain usable images reliably.
Tests of Optic Nerve Function
Frequency Doubling Technology (FDT)
Frequency doubling technology uses a perimeter that takes advantage of an alternative visual stimulus to assess the visual field. It presents flickering stimuli of varying contrast in various locations. The FDT perimeter was the first instrument using this technology. It is small, portable, and can be administered in a screening mode in 45 to 90 seconds. The more recent instrument using this technology is the Humphrey Matrix, which uses smaller targets and has increased the number of locations tested in the visual field. The FDT is smaller than the Humphrey Matrix, but both are relatively portable and technicians can be trained quickly to operate these instruments.
Goldmann Applanation Tonometry (GAT)
Tonometry is the measurement of intraocular pressure (IOP). Applanation tonometry indirectly assesses the IOP by measuring the pressure required to flatten a certain area of the cornea. The Goldmann applanation tonometer uses a standard probe and is the current standard method to measure IOP. The cornea must be anesthetized with an eyedrop. The instrument is mounted on a biomicroscope. Most biomicroscopes are not portable, and skilled training is needed for a technician or clinician to perform tonometry.
Noncontact Tonometry
Noncontact tonometry, also called air-puff tonometry, uses a rapid pulse of air to flatten the cornea. The IOP is estimated by an electro-optical system based on the time needed for the jet of air to flatten the cornea. It takes less time to flatten a soft eye (low IOP) than a hard eye (high IOP). The eye does not need to be anesthetized. Although the pulse is very rapid, patients frequently are startled by this test. Training to operate the instrument is easy, and the table-mounted instrument can be transported when necessary.
Standard Automated Perimetry (SAP)
A perimeter can measure the visual field of an eye in a systematic way by presenting light stimuli of varying intensity at various locations. From the point of fixation, both the width and sensitivity of the visual field can reveal defects typical of glaucoma optic nerve damage. The size and brightness of the light target are varied at multiple locations, and the subject is asked to respond if the image is seen. The resultant score is a critical tool in both the diagnosis and monitoring of the progression of glaucoma. SAP uses a white light stimulus on a white background to determine threshold values. Two instruments in wide use are the Humphrey field analyzer (HFA) and the Octopus. An alternative method of assessing the visual field is short-wavelength automated perimetry (SWAP), which uses a blue stimulus on a yellow background and is thought to be more sensitive for detecting early glaucoma. These instruments are all automated and administered by a technician after a short training time. Because it is subjective, perimetry can be fatiguing for the patient to perform. Furthermore, all devices are large enough to require a tabletop, although some are small enough to be reasonably portable.
Comparators/Reference Standards
KQs 1, 2, 4, 5, and 6 explore comparisons of the interventions mentioned above (conducted alone or in any possible combination as a part of a screening-based program) to no screening program (including usual care, case finding, and referral) and to different screening-based programs (above tests conducted alone or in any possible combination). KQ3 explores comparisons of screening/diagnostic tests to the reference standards of confirmed OAG at the time of followup or OAG requiring treatment (diagnosed by an ophthalmologist using objective assessments). The diagnosis should have included a clinical examination with measurement of IOP, assessment of the visual field, and assessment of the optic nerve head and/or RNFL or review of disc photographs. We considered other methods to confirm diagnosis as defined by included studies whenever the examinations/tests were specified in the report. We acknowledge that there is no consensus on the gold standard test or combination of tests for the identification of patients with OAG. We adapted the reference standards for KQ3 from a diagnostic test accuracy review conducted by Burr et al. (2007).7
Outcomes
KQ1
Primary Outcome
We identified studies that reported the proportion of participants with moderate, severe, and profound visual impairment (as defined in the International Classification of Diseases, Clinical Modification, 9th Revision8). We also considered other measurements of visual impairment as defined by included studies.
Secondary Outcome
We considered visual acuity outcomes (e.g., mean visual acuity or proportion of participants in prespecified visual acuity categories) reported in the included studies and as measured with Snellen or any other valid chart that yields scores that can be converted to Snellen fractions or logarithm of the Minimum Angle of Resolution (logMAR) values.
KQ2
We identified studies that reported the participants’ mean total or relevant item/subscale scores as measured by any validated questionnaire (e.g., National Eye Institute Visual Function Questionnaire) to compare the following patient-reported outcomes among the treatment groups of interest:
- Vision-related quality of life (vision-related functional decrement compared with individuals without eye or vision problems, as well as the impact of functional loss on activities of daily living)—primary outcome
- Patient satisfaction—secondary outcome
KQ3
To calculate sensitivity and specificity, we extracted the number of participants in the following categories: true positives, true negatives, false positives, and false negatives. We also included studies that reported sensitivity, specificity, or area under the receiver operating characteristic curve (AUC).
KQ4
We extracted the mean IOP to analyze the differences between/among the groups of interest.
KQ5
We compared the proportion of participants with progressive optic nerve damage, as defined by included studies and as observed via fundus photography or other imaging of the posterior pole, and the proportion of participants with progression of visual field loss as defined by included studies.
KQ6
We recorded the proportion of participants experiencing the following adverse events (adapted from the U.S. Preventive Services Task Force, www.ahrq.gov/clinic/uspstf05/glaucoma/glaucrs.htm) for each group of interest:
- Corneal abrasions
- Distortion of sense of taste (due to anesthetic use)
- Examination apprehension
- Eye irritation
- Harms related to overdiagnosis
- Infection
- Psychological effects related to a glaucoma diagnosis or misdiagnosis
We also planned to report other harms as reported in included studies. We note that different screening and followup methods may result in different harms.
Timing of Outcome
We assessed outcomes for KQs 1, 2, 4, and 5 at 1 year of followup and at annual intervals thereafter. There was no minimum length of followup for outcomes related to KQs 3 and 6.
Setting
Settings for this review included community screenings, non-eye-care health provider settings, eye-care provider clinical settings (ophthalmologists and optometrists), and telemedicine.
Search Strategy
We searched the following databases for primary studies: MEDLINE®, Embase, LILACS (Latin American and Caribbean Literature on Health Sciences), and CENTRAL (the Cochrane Central Register of Controlled Trials). We developed a search strategy for MEDLINE, accessed via PubMed, based on an analysis of the medical subject heading (MeSH) terms and text words of key articles identified a priori. We adapted this search strategy for searches of Embase (using EMTREE terms), CENTRAL, and LILACS. We searched the literature without imposed language, sample size, or date restrictions. We searched relevant systematic reviews to identify any additional studies that should be included. We searched from the beginning of each database through October 6, 2011.
We also conducted a search in MEDLINE and CENTRAL for systematic reviews that addressed the KQs of interest. The search included the topic strategy (noted in Appendix A of the full report) combined with the term “AND systematic[sb]” and was limited to systematic reviews published from 2009 to 2011. We searched MEDION (www.mediondatabase.nl) for related diagnostic accuracy reviews (KQ3). The search for systematic reviews was conducted on March 2, 2011.
We screened an existing database of eye and vision systematic reviews prepared by Li (2010) to identify relevant OAG systematic reviews published prior to 2009.9 Li searched MEDLINE, Embase, and CENTRAL from inception to September 2009, and two reviewers screened titles, abstracts, and full-text manuscripts to identify eye and vision systematic reviews.
Abstract Screening
We developed an abstract screening form. All investigators pilot tested the form using a set of candidate abstracts identified from the electronic searches. We screened potentially relevant citations (primary studies and systematic reviews) via the Web-based systematic review software DistillerSR (http://systematic-review.net/). All citations identified by the search strategies were uploaded to DistillerSR. Two reviewers independently assessed titles and abstracts resulting from the literature searches according to the inclusion criteria. We classified the titles and abstracts as “include,” “exclude,” or “unsure.” We resolved disagreements about eligibility through discussion among reviewers. We initially reviewed for inclusion non-English-language articles with English abstracts but decided to exclude all non-English articles, as we were unable to identify appropriate translation services for all non-English abstracts and/or the full text of potentially eligible articles prior to the start of full-text screening.
Full-Text Screening
Two reviewers independently applied the same inclusion criteria used during abstract screening. Citations tagged as “unsure” by both reviewers, “unsure” by one reviewer and “include” by the other, or “include” by both reviewers were promoted to full-text screening. We excluded non-English-language articles from further consideration at this stage. We resolved any disagreements regarding inclusion through discussion between reviewers, or, as needed, among all investigators during a team meeting.
Data Abstraction
Data abstraction forms were designed and pilot tested. One reviewer extracted descriptions of the study, including details about the population, devices/tests, and outcomes of interest, using the systematic review software DistillerSR. A second reviewer verified the data. We resolved disagreements through discussion.
Risk-of-Bias Assessment
We used the Cochrane Collaboration’s tool for assessing the risk of bias of randomized and quasi-randomized trials. Two reviewers assessed the included studies for sources of systematic bias according to the guidelines in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions and evaluated the studies for the following criteria: sequence generation and allocation concealment (selection bias); masking of participants, study investigators, and outcome assessors (detection bias); incomplete outcome data (attrition bias); selective outcome reporting (reporting bias); and other sources of bias.10 Masking of investigators and participants may not have been possible with some of the tests being examined but was noted when mentioned. We reported judgments for each criterion as “low risk of bias,” “high risk of bias,” or “unclear risk of bias (information is insufficient to assess).” The two reviewers resolved disagreements through discussion.
Two reviewers assessed the methodological rigor of observational studies using a modified version of the Newcastle Ottawa Scale.11 The Newcastle Ottawa Scale includes domains to assess the quality of study group selection (representativeness, selection, case definitions); comparability of cohorts/cases and controls on the basis of the design or analysis; and ascertainment of exposure(s) or outcome(s), adequacy of followup, nonresponse rate, and financial or other conflicts of interest. Each item query required a “yes,” “no,” or “unable to determine/not reported” response.
For KQ3, we used the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) checklist, which is a specific risk-of-bias assessment for diagnostic accuracy studies.12 The QUADAS tool includes 14 items that evaluate numerous domains, including representativeness, inclusion/exclusion criteria, choice of reference standard, masked interpretation of results of tests and reference standard, and study withdrawal. We reported judgments for each checklist item as “yes,” “no,” or “unclear.”
We used a tool adapted by Li (2010) from the Critical Appraisal Skills Program, Assessment of Multiple Systematic Reviews, and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement to assess the methodological quality of systematic reviews.9 We used the following criteria, adapted from Li, to determine which were of sufficient quality to be considered for inclusion in this review: comprehensive search for primary studies (searches of more than one bibliographic database), inclusion of a risk-of-bias assessment of primary studies, and conduct of appropriate analytic methods for meta-analyses (no pooled-arm analysis).
Rating of Evidence
We assessed the quantity, quality, and consistency of the body of available evidence addressing KQ1 through KQ6. We used an evidence grading scheme recommended by the Grading of Recommendation Assessment, Development and Evaluation (GRADE) Working Group, adapted by AHRQ in the Methods Guide for Effectiveness and Comparative Effectiveness Reviews (www.effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productid=328) and recently published in the Journal of Clinical Epidemiology.13,14
We considered the strength of the study designs, with randomized controlled trials as the highest level of evidence, followed by comparative observational studies. Whenever an outcome was evaluated by at least one RCT, and possibly observational studies as well, we graded the RCT and also the quality of the observational studies. If an outcome was evaluated by only one or by no RCT, our evidence grade was based on the single RCT (if any) and the best available observational study.
We assessed the quality and consistency of the best available evidence, including assessments of the risk of bias in relevant studies, as well as aspects of consistency, directness, and precision, as described in the Methods Guide for Effectiveness and Comparative Effectiveness Reviews and by Owens et al. (2010).14 For each outcome of interest, two reviewers graded the major outcomes for each KQ, and then the entire team discussed their recommendations and reached consensus.
Data Synthesis
When we identified existing high-quality systematic reviews that addressed the KQs, we cited these reviews as evidence and did not abstract and synthesize data from the primary studies. For interventions (screening and diagnostic tests), comparisons, and outcomes that were not covered in systematic reviews, and to update systematic reviews, we abstracted evidence from primary studies, including those that had been published or identified after the date of the last search conducted for the systematic review. We followed the recommendations of Whitlock et al. (2008) for incorporating systematic reviews in complex reviews and provided a narrative summary of the review methods (i.e., inclusion/exclusion criteria, search strategy, statistical methodology) and findings (i.e., number of studies included, quantitative and qualitative results). Similarly, in the instance of multiple reviews, we evaluated the consistency across reviews addressing the same KQ.15
Results
The electronic search of MEDLINE and CENTRAL identified 64 systematic review titles and abstracts. The Li 2010 database included 105 additional systematic review titles and abstracts. We excluded 167 of the 169 systematic review titles and abstracts for the following reasons: did not address any of the KQs, narrative summary only, could not retrieve full text to assess, similar inclusion criteria but date of search for studies older than another included systematic review on the same topic, and duplicate reference to an included systematic review. We identified two systematic reviews for inclusion.7,16 One systematic review (Burr et al., 2007)7 addressed the diagnostic test accuracy of candidate screening tests for the detection of OAG (KQ3), and the second review (Hatt et al., 2006)16 addressed the question of whether screening-based programs prevent optic nerve damage due to OAG when compared with no screening (KQ5).
The electronic searches conducted for concurrent CERs of screening and treatment for OAG identified a total of 4,960 primary study titles and abstracts. After removing duplicate citations, conference abstracts, and book chapters (n = 1,083), we reviewed 3,877 titles and abstracts. We retrieved the full text of 652 articles and assessed the studies for inclusion in this review. We included 83 primary studies that addressed the diagnostic accuracy of candidate screening tests for the detection of OAG that were not included in the Burr et al., 2007, systematic review (KQ3) because the investigators examined newer technologies or the manuscript was published after December 6, 2005. We did not identify any primary studies eligible for inclusion for any other KQ.
Because there was appreciable variability in devices, parameters, thresholds, and measurement of outcomes reported in the primary studies of interest, we did not combine the results using meta-analysis and instead present a narrative summary, with particular emphasis on studies that identified early disease and/or examined newer and more frequently reported technologies. As we are unable to determine which parameters are most important for identifying persons with OAG and as our reported results would have been limited to a few parameters in a subset of studies, we chose to discuss, as appropriate, the full complement of device parameters and thresholds as reported in the included studies. We summarize, where possible, the magnitude of validity across all parameters of interest for devices considered in this report.
Of the devices that were included in the Burr et al. (2007) review, the following were also identified from the search of the literature conducted for this report: HRT II, optic disc photography, RNFL photography, FDT, HFA, GAT, and noncontact tonometry. As there are differences in the eligibility criteria for the current report and the Burr et al. review, including the devices, outcomes, and comparisons of interest, we chose not to undertake an update of the quantitative estimates of sensitivity and specificity from the Burr et al. review for the devices that were common to both reviews.
KQ1
We did not identify any study that addressed whether participation in an OAG screening-based program leads to less visual impairment when compared with no screening or another screening-based program.
KQ2
We did not identify any study that addressed whether participation in an OAG screening-based program leads to improvements in patient-reported outcomes when compared with no screening or another screening-based program.
KQ3
Evidence From Systematic Reviews
Burr et al. (2007) conducted a diagnostic test accuracy review of candidate diagnostic and screening tests for OAG.7 In summary, the investigators included 40 studies totaling more than 48,000 participants 40 years of age and older and those at high risk for the development of OAG based on demographic characteristics or comorbidities. The focus was on studies of participants likely to be encountered in a routine screening setting. Tests of optic nerve structure, optic nerve function, and IOP were included and compared with other individual or combination tests. The primary reference standard was confirmation of OAG at followup examination. Also considered was diagnosis of OAG requiring treatment. Prespecified outcomes were measures related to sensitivity, specificity, harms, acceptability, and reliability. There was significant statistical heterogeneity among the included studies for the majority of the tests, with the exception of optic disc photography (sensitivity), HRT II (sensitivity and specificity), and FDT C-20-1 (sensitivity). The authors also note that no studies were at low risk of bias for all of the modified QUADAS domains examined. A small subset of eight studies was judged to have higher quality, as the study investigators enrolled participants who were representative of a screening/diagnostic setting (low risk of spectrum bias). As well, these studies were at low risk of verification bias (both partial and differential), test bias, and diagnostic review bias.
Detailed Analysis of Primary Studies
We undertook a search for additional primary studies, as described in the Methods section, to address the diagnostic accuracy of candidate screening tests, and identified 83 studies.
With respect to the risk of bias of included primary studies, 68 percent of the included studies were at high risk of spectrum bias, as the study investigators enrolled participants who were not representative of those who would receive the test in practice (i.e., healthy volunteers compared with participants with known glaucoma). Six percent of the studies were at high risk of differential verification bias because the study investigators applied a different reference standard to a subset of participants enrolled in the study. A low percentage (2 percent) were at high risk of incorporation bias, but due to the lack of detail in the descriptions of the reference standard, it was unclear whether the reference standard and candidate tests were independent of each other in 12 percent of the included studies.
With respect to masking of study personnel interpreting the results of the reference standard and candidate tests, the candidate test(s) were interpreted without knowledge of the reference standard result in 29 percent of the included studies, and the reference test interpreted without knowledge of the candidate test(s) in 44 percent of included studies, but we judged these domains to be unclear in 54 percent and 48 percent of the included studies, respectively. Forty-eight percent of the studies did not include an explanation of withdrawals from the study, and 46 percent of the studies reported the number of uninterpretable test results.
Tests of Optic Nerve Structure
Heidelberg Retina Tomograph II
Evidence From Burr et al., 2007
HRT II was a diagnostic test of interest in three studies. Using the common criterion of one or more results that are borderline or outside normal limits, the pooled sensitivity was 86 percent (95% credible interval [CrI], 55 to 97) and the pooled specificity was 89 percent (95% CrI, 66 to 98).
Evidence From Primary Studies
Seventeen primary studies included measures of diagnostic accuracy for HRT II.17–33 Naithani et al. (2007)25 and Uysal et al. (2007)27 specifically focused on detecting early or moderate glaucoma.
Naithani et al. (2007) enrolled 60 participants with glaucoma (30 with early defects and 30 with moderate visual field defects) and 60 healthy volunteers.25 AUC values were reported to be in the range of 0.474 (disc area ratio parameter) to 0.852 (vertical cup-to-disc ratio parameter).
Uysal et al. (2007) enrolled 70 participants with early or moderate glaucoma and 70 healthy volunteers.27 The range of sensitivity across 12 parameters was from 47.1 percent (RNFL cross-sectional area) to 74.3 percent (linear cup/disc area ratio), and the range of specificity was from 47.1 percent (mean RNFL thickness) to 71.4 percent (cup shape measure).
The remaining 15 studies explored comparisons of HRT II with other devices, such as the GDx with VCC (variable corneal compensation), OCT, HRT III, and FDT. Overall, HRT II was found not to perform as well as GDx VCC, OCT, or FDT. HRT II and HRT III were found to have a similar diagnostic profile. Three of the included studies concluded that HRT II was not an appropriate tool for population-based glaucoma screening studies.
Heidelberg Retina Tomograph III
Evidence From Primary Studies
Eleven studies examined the diagnostic accuracy of HRT III. 23,24,28,34–41 Reddy et al. (2009) identified 81 participants with early visual field loss (out of 247 participants with glaucoma) and 142 healthy volunteers. Early visual field loss was defined as a mean deviation less than 5dB.36 The sensitivity of the Glaucoma Probability Score for distinguishing eyes with early field loss from healthy eyes was 67.9 percent, and that of the Moorfields Regression Analysis was 71.9 (at a fixed specificity of 92 percent). The investigators concluded: “Moorfields Regression Analysis and Glaucoma Probability Score have similar ability to detect glaucomatous changes, and typically agree. The relative ease and sensitivity of the operator-independent Glaucoma Probability Score function of the HRT III may facilitate glaucoma screening.”
Badala et al. (2007) compared four imaging methods for their ability to distinguish early glaucoma from healthy eyes.40 Forty-six eyes from 46 participants with early OAG and 46 eyes from healthy volunteers were enrolled. Sensitivity (parameter: reference height) ranged from 4 to 70 percent (Frederick S. Mikelberg discriminant function and Reinhard O. W. Burk discriminant function) when holding the specificity of the test constant at 95 percent.
Ophthalmoscopy
Evidence From Burr et al., 2007
Burr et al. (2007) included seven studies addressing the diagnostic accuracy of ophthalmoscopy. Using a common cutoff point of a vertical cup-to-disc ratio greater than or equal to 0.7, pooled sensitivity for the five studies with this common criterion was 60 percent (95% CrI, 34 to 82 percent), and specificity was 94 percent (95% CrI, 76 to 99). The diagnostic odds ratio (DOR) was 25.7 (95% CrI, 5.79 to 109.50), suggesting a 26-fold higher odds of a positive test among those with glaucoma than those without glaucoma.
Optical Coherence Tomography (OCT)
Evidence From Primary Studies
Of the 47 included studies that investigated the diagnostic accuracy of OCT, 18,25,26,29–32,34,40,42,43–79 34 considered the Stratus OCT, 10 included the Cirrus OCT, 6 considered the RTVue OCT, 2 included the Spectralis OCT, 2 examined the OTI OCT, and 1 included the OTI Spectral OCT/SLO. Across the 34 studies that examined the Stratus OCT, all were at high risk of spectrum bias because those with known disease as well as those with healthy eyes were enrolled in the studies. The sample size ranged from 26 to 95 participants with glaucoma or suspected glaucoma and 37 to 128 healthy volunteers, with one study also enrolling 130 participants with ocular hypertension. For the parameter average RNFL thickness, the range of sensitivity was 24 to 96 percent, suggesting appreciable heterogeneity among the studies. The range of specificity was 66 to 100 percent.
Optic Disc Photography
Evidence From Burr et al., 2007
There were six studies of optic disc photography. The range of sensitivity was from 65 to 77 percent, and the range of specificity was from 59 to 98 percent.
Evidence From Primary Studies
We included two studies of the diagnostic accuracy of optic disc photography33,80 and one study of cup-to-disc ratio measurement as measured by an ophthalmologist using a slit-lamp biomicroscope and 78 Diopter lens.81 Danesh-Meyer et al. (2006) included participants with OAG as well as glaucoma suspects and healthy volunteers.33 The AUC (comparison of those deemed to have glaucoma and borderline disease vs. normal) was 0.84 (95% confidence interval [CI], 0.74 to 0.92) for the cup-to-disc ratio and 0.95 (95% CI, 0.80 to 0.98) for the Disc Damage Likelihood Score, suggesting that the Disc Damage Likelihood Score is a more effective means of discriminating people with and without disease. The diagnostic accuracy of cup-to-disc ratio measurement from the Francis et al. (2011) study81 is described in the section on FDT C-20 perimetry.
RNFL Photography
Evidence From Burr et al., 2007
The common cutoff point for the four included studies was diffuse and/or localized defect observed on RNFL photographs. The pooled diagnostic odds ratio was 23.1 (95% CrI, 4.41 to 123.50), and the pooled sensitivity and specificity were 75 and 88 percent, respectively.
Evidence From Primary Studies
Two studies examined the accuracy of RNFL photography.60,82 Hong et al. (2007) analyzed RNFL photographs of 72 glaucoma and 48 healthy participants.83 Results showed the RNFL defect score II, with an AUC of 0.75 (p < 0.001), was the best parameter for discriminating early glaucoma from healthy eyes (sensitivity, 58.3 percent; specificity, 95.8 percent).
Medeiros et al. (2004) compared RNFL photography with the GDx with VCC in 42 participants with OAG, 32 persons suspected of having OAG, and 40 healthy volunteers.82 The sensitivities of the global RNFL score were 36 and 81 percent, respectively, for fixed specificities of 95 and 80 percent. At a fixed specificity of 95 percent, the sensitivity of the Nerve Fiber Indicator was 71 percent versus the 36 percent reported above for red-free photos. Overall, the global RNFL score determined from red-free photos did not perform as well as scanning laser polarimetry. The AUC was 0.91 for the GDx with VCC Nerve Fiber Indicator versus 0.84 for the global RNFL score.
Scanning Laser Polarimetry (GDx)
Evidence From Primary Studies
Twenty-seven studies included an investigation of the GDx with VCC.18,20,22,26,29–32,40,45,58,59,61,64,71,77,78,80,82–90 The aim of eight studies was to discriminate early glaucoma from no disease.18,20,40,45,83,85,88 In the studies that focused on early OAG, the range of sensitivity across all comparisons and cutoffs for the most frequently reported parameter—Temporal, Superior, Nasal, Inferior, Temporal average—was 29.8 to 81.63 percent. Specificity was fixed at 80, 90, or 95 percent in three studies, and the lowest reported specificity was 66.36 percent. The range in sensitivity for the nerve fiber indicator parameter across all comparisons and cutoffs was from 28.3 to 93.3 percent. The lowest specificity reported was 52.9 percent or was fixed at 80, 90, or 95 percent.
Three studies examined the GDx with enhanced corneal compensation (ECC).59,86,87 The sample sizes of the included studies ranged from 63 to 92 glaucoma participants and 41 to 95 healthy volunteers. Medeiros et al. (2007) compared the AUCs for GDx with VCC and GDx with ECC, and reported that GDx with ECC performed significantly better than GDx with VCC for the parameters Temporal, Superior, Nasal, Inferior, Temporal average, Superior average, and Inferior average (p = <0.01).86 Sehi et al. (2007)59 and Mai et al. (2007)87 concurred with Medeiros et al. (2007) that imaging with ECC appears to improve the ability to diagnose OAG.
Tests of Optic Nerve Function
FDT (C-20-1) Perimetry
Evidence From Burr et al., 2007
The pooled sensitivity and specificity results for the three studies that included FDT (C-20-1) perimetry and the common diagnostic criterion of one abnormal test point were high (92 and 94 percent, respectively).
Evidence From Primary Studies
Four studies discussed the accuracy of FDT C-20 perimetry.18,81,91,92 Pueyo et al. (2009) enrolled 130 participants with ocular hypertension and 48 healthy volunteers.18 Using a cutoff of a cluster of at least four points with a sensitivity outside 95 percent normal limits, or three points outside 98 percent normal limits, or at least one point outside 99 percent normal limits, investigators determined the sensitivity of FDT to be 31.25 percent and its specificity 72.9 percent among the subset of 32 participants with glaucomatous optic neuropathy (of the 130 with ocular hypertension). The investigators concluded that FDT might not be an ideal test for participants with early defects.
Salim et al. (2009) enrolled 35 participants with known OAG and 35 age- and sex-matched controls with no evidence of glaucoma. Investigators used FDT, noncontact tonometry, and a questionnaire individually and in all possible combinations to determine the accuracy of single and combination tests.91 FDT’s sensitivity was 58.1 percent and its specificity was 98.6 percent. Overall, FDT was determined to be the best among the candidate single and combination tests in the study, despite fair sensitivity for detecting OAG.
Pierre-Filho et al. (2006) enrolled glaucoma patients who had never experienced perimetry prior to the study.92 The investigators reported that 21 (32.8 percent) of the 64 participants with glaucoma were identified as having early disease, but data were not provided for this subgroup. Sensitivity and specificity were 85.9 and 73.6 percent, respectively, for the presence of at least one abnormal location and 82.8 and 83 percent, respectively, for two or more abnormal locations, regardless of severity.
Francis et al. (2011) conducted population-based screening of 6,082 Latinos age 40 years and older as part of the Los Angeles Latino Eye Study (LALES) to determine the diagnostic accuracy of candidate screening tests performed alone or in combination.81 Participants completed Humphrey Visual Field testing in addition to FDT C-20-1, GAT, and central corneal thickness and cup-to-disc ratio measurements. Diagnostic test accuracy outcomes were assessed for the general population as well as high-risk subgroups, defined as persons who were 65 years and older, those with a family history of glaucoma, and persons with diabetes. Of the 6,082 participants screened, 4.7 percent (286) were diagnosed as having OAG. Based on three glaucoma diagnosis definitions (glaucomatous optic nerve appearance, glaucomatous visual field loss, glaucomatous optic nerve and visual field loss), the test parameters vertical cup-to-disc ratio ≥ 0.8 and Humphrey Visual Field (HVF) false negatives ≥ 33 percent had the highest specificity, regardless of the definition of glaucoma (98 percent). HVF mean deviation < 5 percent had the highest sensitivity (78 percent) using the definition of optic nerve defects only, while the HVF glaucoma hemifield test had the highest sensitivity under the other two definitions (90 percent for glaucomatous visual field loss and 90 percent for both field loss and optic nerve damage). Specific results for the FDT C-20-1 were as follows (sensitivity/specificity, definition of glaucoma): 59 percent/79 percent, glaucomatous optic nerve appearance only; 68 percent/80 percent, glaucomatous visual field loss only; 67 percent/79 percent, both glaucomatous optic nerve appearance and visual field loss. The investigators reported similar results when high-risk subgroups were analyzed and concluded that “these results suggest that screening of high-risk groups based on these criteria may not improve over screening of the general population over age 40.”81
FDT (C-20-5) Perimetry
Evidence From Burr et al., 2007
Five studies of FDT (C-20-5) with significant heterogeneity using the common cutoff point of one abnormal test point were included. The range of sensitivity was 7 to 100 percent; the specificity range was 55 to 89 percent.
FDT 24-2 Perimetry
Evidence From Primary Studies
Five studies examined the diagnostic accuracy of FDT 24-2 threshold tests using the Humphrey Matrix Perimeter.64,93–96 All studies included participants with known glaucoma and healthy volunteers, and we judged these studies to be at high risk of spectrum bias. The range of sample size was 25 to 174 glaucomatous eyes and 15 to 164 healthy eyes. Sensitivities and specificities were reported for the parameters mean deviation, pattern standard deviation, and glaucoma hemifield test outside of normal limits. There was appreciable heterogeneity in the estimates of sensitivity at 80 percent, 90 percent, and 95 percent specificity that may be attributed to a number of factors, including different patient populations and variations in cutoff points. The sensitivity was 55 percent for the mean deviation and 94 percent at 80 percent fixed specificity.94,95 Tafreshi et al. (2009) and Leeprechanon et al. (2007) reported 39 and 87 percent at 90 percent fixed specificity, and 32 and 82 percent at fixed 95 percent specificity, respectively.93,95 Sensitivity and specificity for pattern standard of deviation (PSD) and glaucoma hemifield test are reported with their cutoff points in the evidence tables in Appendix C of the full report.
Bagga et al. (2006)64 and Burgansky-Eliash et al. (2007)96 reported the AUC for the mean deviation parameter (0.69 for both studies with p < 0.04 and 95% CI, 0.564 to 0.815, respectively). The AUCs for PSD were 0.66 (p = 0.09)64 and 0.733 (95% CI, 0.618 to 0.848).96
FDT 30-2 Perimetry
Evidence From Primary Studies
Two studies discussed the detection of early glaucoma using the FDT 30-2 threshold test with the Humphrey Matrix Perimeter.60,83 Both Hong, Chung, Hong, et al. (2007)60 and Hong, Ahn, Ha, et al. (2007)83 enrolled OAG participants with early visual field loss and healthy controls. The mean deviation and PSD were judged to be good parameters for distinguishing between eyes with early disease and eyes with no known defects. The mean deviations were 0.795 and 0.750 and the PSDs were 0.808 and 0.934 for Hong, Chung, Hong, et al. and Hong, Ahn, Ha, et al., respectively. Both study groups, however, determined that the best parameter for distinguishing eyes with early glaucoma from healthy eyes was the number of points that have p less than 5 percent in the pattern deviation plot, with an AUC of 0.985 (95% CI, 0.943 to 0.998) in Hong, Chung, Hong, et al. and 0.990 (p < 0.001) in Hong, Ahn, Ha, et al.
FDT N-30 Perimetry
Evidence From Primary Studies
Four studies examined the accuracy of the FDT N-30 threshold test.20,94,97,98 Zeppieri et al. (2010) focused on the detection of early glaucoma among a sample of 75 participants with OAG, 87 with ocular hypertension, 67 with glaucomatous optic neuropathy, and 90 healthy volunteers.20 At the best cutoff of less than −0.78, the sensitivity of the mean deviation parameter was 61.3 percent and the specificity was 73.7 percent for distinguishing early OAG from healthy eyes. At the best cutoff of greater than 3.89, the sensitivity of the PSD was 76.0 percent and the specificity was 87.8 percent. Salvetat et al. (2010) focused on the detection of early disease among a sample of 52 participants with early OAG and 53 healthy volunteers.98 The sensitivity of mean deviation for distinguishing early OAG from healthy eyes at the best cutoff (less than −1.12) was 67 percent and the specificity was 74 percent. At the best cutoff of greater than 3.97, the sensitivity of the parameter PSD was 96 percent and the specificity was 85 percent.
Goldmann Applanation Tonometry
Evidence From Burr et al., 2007
At the common cutoff point of IOP greater than 20.5–22 mm Hg, nine studies with significant heterogeneity reported sensitivity in the range of 10 to 90 percent and specificity in the range of 81 to 99 percent.
Evidence From Primary Studies
Two studies64,81 included examination of GAT. Bagga et al. (2006) compared the ability of various tests of structure and function to discriminate healthy eyes (n = 22) from eyes with known glaucomatous optic neuropathy (n = 25).64 The AUC for IOP, as measured by GAT, was 0.66 (p = 0.05).
The methods of the Francis et al. (2011) study (LALES)81 are discussed in the FDT C-20 section of the full report. The specific sensitivity and specificity values for GAT using a cutoff of ≥ 21 mm Hg for the three definitions of glaucoma were as follows (sensitivity/specificity, definition of glaucoma): 21 percent/97 percent, glaucomatous optic nerve appearance only; 23 percent/97 percent, glaucomatous visual field loss only; 24 percent/97 percent, both glaucomatous optic nerve appearance and visual field loss.
Humphrey Visual Field Analyzer
Evidence From Primary Studies
Ten studies examined the diagnostic accuracy of the HFA. Of these, six examined HFA Short Wavelength Automated Perimetry;18,44,64,93,97,99 two tested HFA-SAP, (SAP)-SITA, and HFA SAP-Full Threshold (FT);93,97 four examined HFA-SITA-Standard;33,90,92,96 and one tested the HFA SITA-Fast protocol.92 The HFA Short Wavelength Automated Perimetry testing protocol (the most frequently reported) included 25 to 286 participants with glaucoma and 22 to 289 healthy volunteers across the six included studies. Sensitivity across all comparisons and cutoffs for the mean deviation ranged from 25.9 to 83 percent. Specificity ranged from 80 to 95.2 percent. Cutoff points ranged from −5.42 to −11.06 dB.
Noncontact Tonometry
Evidence From Burr et al., 2007
One study reported a sensitivity of 92 percent and specificity of 92 percent using the criterion of IOP greater than 21 mm Hg.
Evidence From Primary Studies
Salim et al. (2009) included noncontact tonometry, individually and in all possible combinations, with other measures of structure and function to determine the accuracy of single and combination tests.91 IOP, as measured by noncontact tonometry, was found not to be a very sensitive test for detecting glaucoma (sensitivity 22.1 percent). The investigators acknowledge that use of topical medications by the glaucoma participants could limit the ability to identify those with disease.
Oculokinetic Perimetry
Evidence From Burr et al., 2007
Four studies were included that examined the diagnostic accuracy of oculokinetic perimetry. The common criterion varied in description, but is best described as one or more points missing. The odds of a positive test were 57 times as high (DOR, 57.54) for those with glaucoma as for those without glaucoma (95% CrI, 4.42 to 1585.00). The pooled sensitivity and specificity were 86 and 90 percent, respectively.
SAP Suprathreshold Test
Evidence From Burr et al., 2007
Nine studies, including the Baltimore Eye Survey and the Blue Mountains Eye Study, examined the SAP suprathreshold test. Although the sensitivity and specificity were similar for the Baltimore and Blue Mountains studies, there was significant heterogeneity among the included studies. The range in sensitivity was 25 to 90 percent; the range in specificity was 67 to 96 percent.
SAP Threshold Test
Evidence From Burr et al., 2007
Among the five studies analyzed for SAP threshold, both Humphrey 30-2 and 24-2 threshold and Octopus 500 were evaluated. The pooled sensitivity was 88 percent, and specificity was 80 percent for the common cutoff point. (The definition of the common cutoff point differed by included study, but is defined in Burr et al.)
Tendency-Oriented Perimetry
Evidence From Primary Studies
Pierre-Filho et al. (2006) compared frequency doubling technology), tendency-oriented perimetry using the Octopus 301 G1-TOP program, SITA Standard, and SITA Fast in 117 eyes (64 with glaucoma and 53 healthy eyes).92 The Octopus 301 perimeter test was considered abnormal under two conditions: when the mean defect was “> 2dB and/or the loss variance > 6 dB (TOP 1), and…there were at least seven points (three of them contiguous) with a reduction in sensitivity ≥ 5 dB in the corrected comparisons graphic (TOP 2).”92 The sensitivity using definition TOP 1 was 87.5 percent (95% CI, 76.3 to 94.1) and the specificity was 56.6 percent (95% CI, 42.4 to 69.9). With definition TOP 2, the sensitivity was 89.1 percent (95% CI, 78.2 to 95.1) and the specificity was 62.3 percent (95% CI, 47.9 to 74.9).
Direct Comparisons of Candidate Tests
Evidence From Burr et al., 2007
Six studies included comparisons of SAP with optic disc photography, HRT II, FDT, and/or GAT. Burr et al. concluded that sensitivity results at the common cutoff point for each test revealed that SAP performed better than GAT. One of the two studies that addressed the comparison of SAP to GAT reported estimates of sensitivity of 89 percent and 3 to 14 percent, respectively. Specificity values were 73 percent for SAP and 98 to 99 percent for GAT. Burr et al. also concluded that SAP was similar to HRT II. The sensitivity of SAP was 72 percent and the sensitivity of HRT II was 69 percent in one of the two included studies; the specificity for both tests was 95 percent. There was one included study in which the investigators compared SAP with optic disc photography. Optic disc photographs had a similar sensitivity (73 to 77 percent) and specificity (59 to 62 percent) to SAP (sensitivity, 50 to 71 percent; specificity, 58 to 83 percent). In the two studies that included comparisons of SAP with FDT, one study reported similar sensitivity estimates (SAP, 63 to 90 percent; FDT C-20-5, 68 to 84 percent) and similar specificity values (SAP, 58 to 74 percent; FDT C-20-5, 55 to 76 percent).
Based on analyses of the common criterion for each test, test accuracy, combination tests, tests for glaucoma at specific stages, and direct and indirect comparisons of tests, Burr et al. (2007) concluded that optic disc photography, HRT II, FDT, SAP, and GAT were candidates for use in a screening-based program.
Conclusion
Based on the Burr et al. (2007) findings,7 standard automated perimetry was compared with other tests available at that time. SAP had higher sensitivity than Goldmann tonometry, similar sensitivity to HRT, and lower sensitivity than disc photos or FDT. In terms of specificity, SAP performed better than disc photos and FDT, similar to HRT, and worse than Goldmann tonometry.
We identified several additional studies assessing the performance of glaucoma screening tests not included in the Burr et al. review. The studies included newer imaging (GDx, HRT III, OCT) and functional (Short Wavelength Automated Perimetry, new FDT patterns) technologies. However, despite improvements in the technology, it is still not clear that there is any one test or combination of tests suitable for use in glaucoma screening in the general population. Significant barriers to identifying and characterizing potential glaucoma screening tests remain. These barriers include the lack of a definitive diagnostic reference standard for glaucoma and heterogeneity in the design and conduct of the studies. Because of these barriers, the ranges of sensitivities, specificities, and AUCs are large and prevent a coherent synthesis.
KQ4
We did not identify any study that addressed whether participation in an OAG screening-based program leads to reductions in IOP when compared with no screening or another screening-based program.
KQ5
Evidence From Systematic Reviews
Hatt et al. (2006) undertook a systematic review of randomized trials of screening modalities for OAG compared with no screening (including opportunistic case finding and referral). There were no restrictions on included populations.16 The primary outcome of interest was the prevalence of visual field loss, defined as the proportion of participants with a prespecified severity of visual field loss diagnosed by either manual or automated field assessment. Other primary outcomes included the prevalence of optic nerve damage and visual impairment. Electronic searches of five databases, including MEDLINE and CENTRAL, were conducted in 2006 and again in January 2009, but none of the studies that were identified were eligible for inclusion. The review authors acknowledged that RCTs require lengthy followup and are predicated on identifying appropriate candidate tests that may be incorporated into a screening-based program.
Detailed Analysis of Primary Studies
We did not identify any study that addressed whether participation in an OAG screening-based program leads to reductions in visual field loss or optic nerve damage when compared with no screening or another screening-based program.
KQ6
We did not identify any study addressing the harms associated with screening for OAG.
Discussion
The purpose of this Comparative Effectiveness Review was to summarize the evidence linking screening for glaucoma to intermediate and functional health outcomes of treatment. We did not identify evidence to address five of the six KQs of interest, as there were no population-based studies that screened and followed treated or untreated asymptomatic persons with disease and also included a suitable comparison group of early glaucoma patients identified via case finding, referral, or a different screening-based program (Figure A).
The investigators of the evidence report Primary Care Screening for Ocular Hypertension and Primary Open-Angle Glaucoma: Evidence Synthesis, commissioned by the Agency for Healthcare Research and Quality in 2005, found no evidence assessing screening and subsequent treatment of glaucoma in a population setting and concluded that while there was good evidence to suggest that treating early primary open-angle glaucoma is beneficial, based on the lack of evidence regarding screening, more research is needed to address whether screening is “effective in improving vision-specific functional outcomes and health-related quality of life.”6 As our updated search of the literature was unable to identify any evidence linking screening to the prespecified intermediate and functional outcomes, we also conclude that more research is needed to address this question. A randomized controlled trial of glaucoma screening would be the optimal study design, as an RCT design would allow investigators to enroll participants with similar risk profiles and minimize the risk of lead-time bias. The feasibility of an RCT would be contingent, however, on both the identification of sufficiently sensitive and specific tests for screening and diagnosing persons with glaucoma and the establishment of a standard definition for OAG.
A sixth KQ (KQ3) addressed the accuracy of candidate screening/diagnostic tests for glaucoma. After completing a systematic review of 40 included studies and 48,000 participants, Burr et al. (2007)7 concluded that optic disc photography, HRT II, FDT, SAP, and GAT were potential candidates for a screening-based program, but acknowledged that given the “imprecision in estimates from the pooled meta-analysis models for the diagnostic performance of each test it was not possible to identify a single test (or even a group of tests) as the most accurate.”7
Building on the comprehensive evaluation by Burr et al. (2007), we identified 83 additional studies evaluating the diagnostic accuracy of candidate tests published as of October 6, 2011. While there is now more evidence regarding Optical Coherence Tomography, the Heidelberg retina tomograph III, and the GDx scanning laser polarimeters, the ability of these devices to identify glaucoma in a screening setting is not well understood for the same reasons as noted by Burr et al.: the lack of a single diagnostic standard for glaucoma and the high degree of variability in the design and conduct of largely cross-sectional studies of diagnostic accuracy. The risk of bias of diagnostic study designs is an additional concern. Many of the glaucoma diagnostic studies included in this review are at high risk of spectrum bias because the investigators compared healthy volunteers with persons with known glaucoma at the time of screening. Enrolling participants who are not representative of those one reasonably expects to encounter in a screening setting results in biased and inflated estimates of diagnostic performance and limits the generalizability of findings. Incorporation bias is of concern, as the reference standard should not include one or more tests that comprise the candidate tests under investigation. But as noted in Burr et al., incorporation bias is a very complex issue when considering the diagnosis of glaucoma. The tests used to diagnose glaucoma are categorized broadly into tests of optic nerve structure or function. To lessen the risk of incorporation bias, one would have to employ a test of structure as the reference standard if the candidate test was one of function or a test of function as the reference if the candidate test were one of structure. However, to do so assumes that “structural (e.g. optic disc) and functional (e.g. visual field) damage occur simultaneously in glaucoma pathogenesis, whereas there is evidence that disc damage precedes manifest visual field loss.”7
Although we intended to include a discussion of the validity of community and non-eye-care health provider screenings, the studies that met the inclusion criteria were conducted in eye-care provider settings only. Three of the 83 studies included a population-based sample, and the remainder included healthy participants and those with known or suspected glaucoma at the time of screening. Given that the majority of the studies included those with known or suspected disease and that the studies were conducted in eye-care provider settings only, the findings of this Comparative Effectiveness Review are not generalizable to primary care and other non-eye-care settings.
Screening for glaucoma is a difficult problem because it is asymptomatic, has low prevalence, is typically only slowly progressive, and has no agreed-upon standard for diagnosis. These issues, while challenging, might be overcome by a combination of creative thinking with regard to populations amenable to screening and hard work on the necessary studies and diagnostic standards.
References
- 1.
- Quigley HA, Browman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. [PMC free article: PMC1856963] [PubMed: 16488940]
- 2.
- The Eye Diseases Prevalence Research Group. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122:532–8. [PMC free article: PMC2798086] [PubMed: 15078671]
- 3.
- Foster PJ, Buhrmann R, Quigley HA, et al. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–42. [PMC free article: PMC1771026] [PubMed: 11815354]
- 4.
- Quigley HA, Vitale S. Models of open-angle glaucoma prevalence and incidence in the United States. Invest Ophthalmol Vis Sci. 1997;38:83–91. [PubMed: 9008633]
- 5.
- US Preventive Services Task Force. Screening for Glaucoma: Recommendation Statement. Rockville, MD: Agency for Healthcare Research and Quality; 2005. AHRQ Publication No. 04-0548-A. [PubMed: 16342838]
- 6.
- Fleming C, Whitlock E, Beil T, et al. Screening for primary open-angle glaucoma in the primary care setting: an update for the US Preventive Services Task Force. Ann Fam Med. 2005;3(2):167–70. [PMC free article: PMC1466856] [PubMed: 15798044]
- 7.
- Burr JM, Mowatt G, Hernandez R, et al. The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation. Health Technol Assess. 2007;11(41) [PubMed: 17927922]
- 8.
- International Classification of Diseases, Ninth Revision (ICD-9). Centers for Disease Control and Prevention; 2009.
- 9.
- Li T. PhD dissertation. Baltimore: The Johns Hopkins University; 2010. Bridging the gap between evidence generation and clinical decision making.
- 10.
- Higgins JPT, Altman DG. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009). The Cochrane Collaboration; 2009. Chapter 8: Assessing risk of bias in included studies.
- 11.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. [Accessed November 10, 2011]. www
.ohri.ca/programs /clinical_epidemiology/oxford.htm. - 12.
- Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3(25) [PMC free article: PMC305345] [PubMed: 14606960]
- 13.
- Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. [PMC free article: PMC428525] [PubMed: 15205295]
- 14.
- Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions-Agency for Healthcare Research and Quality and the Effective Health-care Program. J Clin Epidemiol. 2010;63(5):513–23. [PubMed: 19595577]
- 15.
- Whitlock EP, Lin JS, Chou R, et al. Using existing systematic reviews in complex systematic reviews. Ann Intern Med. 2008;148:776–82. [PubMed: 18490690]
- 16.
- Hatt S, Wormald R, Burr J. Screening for prevention of optic nerve damage due to chronic open angle glaucoma. Cochrane Database Syst Rev. 2006;(4):CD006129. [PMC free article: PMC8407423] [PubMed: 17054274]
- 17.
- Healey PR, Lee AJ, Aung T, et al. Diagnostic accuracy of the Heidelberg Retina Tomograph for glaucoma: a population-based assessment. Ophthalmology. 2010;117(9):1667–73. [PubMed: 20816247]
- 18.
- Pueyo V, Polo V, Larrosa JM, et al. Ability of optical imaging devices to detect early structural damage in ocular hypertension. Ann Ophthalmol. 2009;41(3–4):150–6. [PubMed: 20214046]
- 19.
- Zheng Y, Wong TY, Lamoureux E, et al. Diagnostic ability of Heidelberg Retina Tomography in detecting glaucoma in a population setting: the Singapore Malay Eye Study. Ophthalmology. 2010;117(2):290–7. [PubMed: 20006907]
- 20.
- Zeppieri M, Brusini P, Parisi L, et al. Pulsar perimetry in the diagnosis of early glaucoma. Am J Ophthalmol. 2010;149(1):102–12. [PubMed: 19800607]
- 21.
- Saito H, Tsutsumi T, Araie M, et al. Sensitivity and specificity of the Heidelberg Retina Tomograph II Version 3.0 in a population-based study: the Tajimi Study. Ophthalmology. 2009;116(10):1854–61. [PubMed: 19660814]
- 22.
- Medeiros FA, Vizzeri G, Zangwill LM, et al. Comparison of retinal nerve fiber layer and optic disc imaging for diagnosing glaucoma in patients suspected of having the disease. Ophthalmology. 2008;115(8):1340–6. [PMC free article: PMC2832850] [PubMed: 18207246]
- 23.
- Ferreras A, Pablo LE, Pajarin AB, et al. Diagnostic ability of the Heidelberg Retina Tomograph 3 for glaucoma. Am J Ophthalmol. 2008;145(2):354–9. [PubMed: 18078851]
- 24.
- De Leon-Ortega JE, Sakata LM, Monheit BE, et al. Comparison of diagnostic accuracy of Heidelberg Retina Tomograph II and Heidelberg Retina Tomograph 3 to discriminate glaucomatous and nonglaucomatous eyes. Am J Ophthalmol. 2007;144(4):525–32. [PMC free article: PMC3928044] [PubMed: 17693382]
- 25.
- Naithani P, Sihota R, Sony P, et al. Evaluation of optical coherence tomography and heidelberg retinal tomography parameters in detecting early and moderate glaucoma. Invest Ophthalmol Vis Sci. 2007;48(7):3138–45. [PubMed: 17591883]
- 26.
- Pueyo V, Polo V, Larrosa JM, et al. Diagnostic ability of the Heidelberg retina tomograph, optical coherence tomograph, and scanning laser polarimeter in open-angle glaucoma. J Glaucoma. 2007;16(2):173–7. [PubMed: 17473725]
- 27.
- Uysal Y, Bayer A, Erdurman C, et al. Sensitivity and specificity of Heidelberg Retinal Tomography II parameters in detecting early and moderate glaucomatous damage: effect of disc size. Clin Experiment Ophthalmol. 2007;35(2):113–8. [PubMed: 17362450]
- 28.
- Burgansky-Eliash Z, Wollstein G, Bilonick RA, et al. Glaucoma detection with the Heidelberg retina tomograph 3. Ophthalmology. 2007;114(3):466–71. [PMC free article: PMC1945822] [PubMed: 17141321]
- 29.
- Shah NN, Bowd C, Medeiros FA, et al. Combining structural and functional testing for detection of glaucoma. Ophthalmology. 2006;113(9):1593–602. [PubMed: 16949444]
- 30.
- Medeiros FA, Zangwill LM, Bowd C, et al. Influence of disease severity and optic disc size on the diagnostic performance of imaging instruments in glaucoma. Invest Ophthalmol Vis Sci. 2006;47(3):1008–15. [PubMed: 16505035]
- 31.
- Medeiros FA, Zangwill LM, Bowd C, et al. Comparison of the GDx VCC scanning laser polarimeter, HRT II confocal scanning laser ophthalmoscope, and stratus OCT optical coherence tomograph for the detection of glaucoma. Arch Ophthalmol. 2004;122(6):827–37. [PubMed: 15197057]
- 32.
- Ferreras A, Polo V, Larrosa JM, et al. Can frequency-doubling technology and short-wavelength automated perimetries detect visual field defects before standard automated perimetry in patients with preperimetric glaucoma? J Glaucoma. 2007;16(4):372–83. [PubMed: 17571000]
- 33.
- Danesh-Meyer HV, Gaskin BJ, Jayusundera T, et al. Comparison of disc damage likelihood scale, cup to disc ratio, and Heidelberg retina tomograph in the diagnosis of glaucoma. Br J Ophthalmol. 2006;90(4):437–41. [PMC free article: PMC1857000] [PubMed: 16547323]
- 34.
- Moreno-Montanes J, Anton A, Garcia N, et al. Comparison of retinal nerve fiber layer thickness values using Stratus Optical Coherence Tomography and Heidelberg Retina Tomograph-III. J Glaucoma. 2009;18(7):528–34. [PubMed: 19745667]
- 35.
- Bozkurt B, Irkec M, Arslan U. Diagnostic accuracy of Heidelberg Retina Tomograph III classifications in a Turkish primary open-angle glaucoma population. Acta Ophthalmol. 2010;88(1):125–30. [PubMed: 19681791]
- 36.
- Reddy S, Xing D, Arthur SN, et al. HRT III glaucoma probability score and Moorfields regression across the glaucoma spectrum. J Glaucoma. 2009;18(5):368–72. [PubMed: 19525726]
- 37.
- Oddone F, Centofanti M, Iester M, et al. Sector-based analysis with the Heidelberg Retinal Tomograph 3 across disc sizes and glaucoma stages: a multicenter study. Ophthalmology. 2009;116(6):1106–11. e1–3. [PubMed: 19376590]
- 38.
- Takmaz T, Can I. Comparison of glaucoma probability score and Moorfields regression analysis to discriminate glaucomatous and healthy eyes. Eur J Ophthalmol. 2009;19(2):207–13. [PubMed: 19253236]
- 39.
- Moreno-Montanes J, Anton A, Garcia N, et al. Glaucoma probability score vs Moorfields classification in normal, ocular hypertensive, and glaucomatous eyes. Am J Ophthalmol. 2008;145(2):360–8. [PubMed: 18045569]
- 40.
- Badala F, Nouri-Mahdavi K, Raoof DA, et al. Optic disk and nerve fiber layer imaging to detect glaucoma. Am J Ophthalmol. 2007;144(5):724–32. [PMC free article: PMC2098694] [PubMed: 17868631]
- 41.
- Ferreras A, Pajarin AB, Polo V, et al. Diagnostic ability of Heidelberg Retina Tomograph 3 classifications: glaucoma probability score versus Moorfields regression analysis. Ophthalmology. 2007;114(11):1981–7. [PubMed: 17445899]
- 42.
- Mansoori T, Viswanath K, Balakrishna N. Quantification of retinal nerve fiber layer thickness in normal eyes, eyes with ocular hypertension, and glaucomatous eyes with SD-OCT. Ophthalmic Surg Lasers Imaging. 2010;41(6):S50–7. [PubMed: 21117601]
- 43.
- Fang Y, Pan YZ, Li M, et al. Diagnostic capability of Fourier-Domain optical coherence tomography in early primary open angle glaucoma. Chin Med J (Engl). 2010;123(15):2045–50. [PubMed: 20819540]
- 44.
- Zhong Y, Zhou X, Cheng Y, et al. Relation between blue-on-yellow perimetry and optical coherence tomography in normal tension glaucoma. Can J Ophthalmol. 2010;45(5):494–500. [PubMed: 20648075]
- 45.
- Pablo LE, Ferreras A, Schlottmann PG. Retinal nerve fibre layer evaluation in ocular hypertensive eyes using optical coherence tomography and scanning laser polarimetry in the diagnosis of early glaucomatous defects. Br J Ophthalmol. 2011 Jan;95(1):51–5. Epub 2010 June 24. [PubMed: 20576777]
- 46.
- Rao HL, Zangwill LM, Weinreb RN, et al. Comparison of different spectral domain optical coherence tomography scanning areas for glaucoma diagnosis. Ophthalmology. 2010;117(9):1692–9. 1699.e1. [PubMed: 20493529]
- 47.
- Leite MT, Zangwill LM, Weinreb RN, et al. Effect of disease severity on the performance of Cirrus spectral-domain OCT for glaucoma diagnosis. Invest Ophthalmol Vis Sci. 2010;51(8):4104–9. [PMC free article: PMC2910643] [PubMed: 20335619]
- 48.
- Li G, Fansi AK, Boivin JF, et al. Screening for glaucoma in high-risk populations using optical coherence tomography. Ophthalmology. 2010;117(3):453–61. [PubMed: 20031231]
- 49.
- Park SB, Sung KR, Kang SY, et al. Comparison of glaucoma diagnostic capabilities of Cirrus HD and Stratus optical coherence tomography. Arch Ophthalmol. 2009;127(12):1603–9. [PubMed: 20008715]
- 50.
- Chang RT, Knight OJ, Feuer WJ, et al. Sensitivity and specificity of time-domain versus spectral-domain optical coherence tomography in diagnosing early to moderate glaucoma. Ophthalmology. 2009;116(12):2294–9. [PubMed: 19800694]
- 51.
- Moreno-Montanes J, Olmo N, Alvarez A, et al. Cirrus high-definition optical coherence tomography compared with Stratus optical coherence tomography in glaucoma diagnosis. Invest Ophthalmol Vis Sci. 2010;51(1):335–43. [PubMed: 19737881]
- 52.
- Sehi M, Grewal DS, Sheets CW, et al. Diagnostic ability of Fourier-domain vs time-domain optical coherence tomography for glaucoma detection. Am J Ophthalmol. 2009;148(4):597–605. [PMC free article: PMC2784699] [PubMed: 19589493]
- 53.
- Yuksel N, Altintas O, Ozkan B, et al. Discriminating ability of optical coherence tomography data in staging glaucomatous damage. Can J Ophthalmol. 2009;44(3):297–307. [PubMed: 19491986]
- 54.
- Sung KR, Kim DY, Park SB, et al. Comparison of retinal nerve fiber layer thickness measured by Cirrus HD and Stratus optical coherence tomography. Ophthalmology. 2009;16(7):1264–70. 1270.e1. [PubMed: 19427696]
- 55.
- Polo V, Larrosa JM, Ferreras A, et al. Retinal nerve fiber layer evaluation in open-angle glaucoma. Optimum criteria for optical coherence tomography. Ophthalmologica. 2009;223(1):2–6. [PubMed: 18849629]
- 56.
- Lu AT, Wang M, Varma R, et al. Combining nerve fiber layer parameters to optimize glaucoma diagnosis with optical coherence tomography. Ophthalmology. 2008;115(8):1352–7. 1357.e1–2. [PMC free article: PMC2756507] [PubMed: 18514318]
- 57.
- Nouri-Mahdavi K, Nikkhou K, Hoffman DC, et al. Detection of early glaucoma with optical coherence tomography (StratusOCT). J Glaucoma. 2008;17(3):183–8. [PubMed: 18414102]
- 58.
- Takahashi H, Chihara E. Impact of diabetic retinopathy on quantitative retinal nerve fiber layer measurement and glaucoma screening. Invest Ophthalmol Vis Sci. 2008;49(2):687–92. [PubMed: 18235015]
- 59.
- Sehi M, Ume S, Greenfield DS. Scanning laser polarimetry with enhanced corneal compensation and optical coherence tomography in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2007;48(5):2099–104. [PubMed: 17460267]
- 60.
- Hong S, Chung W, Hong YJ, et al. Discriminating ability of Humphrey matrix perimetry in early glaucoma patients. Ophthalmologica. 2007;221(3):195–9. [PubMed: 17440283]
- 61.
- Brusini P, Salvetat ML, Zeppieri M, et al. Comparison between GDx VCC scanning laser polarimetry and Stratus OCT optical coherence tomography in the diagnosis of chronic glaucoma. Acta Ophthalmol Scand. 2006;84(5):650–5. [PubMed: 16965496]
- 62.
- Sihota R, Sony P, Gupta V, et al. Diagnostic capability of optical coherence tomography in evaluating the degree of glaucomatous retinal nerve fiber damage. Invest Ophthalmol Vis Sci. 2006;47(5):2006–10. [PubMed: 16639009]
- 63.
- Chen HY, Wang TH, Lee YM, et al. Retinal nerve fiber layer thickness measured by optical coherence tomography and its correlation with visual field defects in early glaucoma. J Formos Med Assoc. 2005;104(12):927–34. [PubMed: 16607450]
- 64.
- Bagga H, Feuer WJ, Greenfield DS. Detection of psychophysical and structural injury in eyes with glaucomatous optic neuropathy and normal standard automated perimetry. Arch Ophthalmol. 2006;124(2):169–76. [PubMed: 16476885]
- 65.
- Leung CK, Chan WM, Yung WH, et al. Comparison of macular and peripapillary measurements for the detection of glaucoma: an optical coherence tomography study. Ophthalmology. 2005;112(3):391–400. [PubMed: 15745764]
- 66.
- Medeiros FA, Zangwill LM, Bowd C, et al. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am J Ophthalmol. 2005;139(1):44–55. [PubMed: 15652827]
- 67.
- Wollstein G, Ishikawa H, Wang J, et al. Comparison of three optical coherence tomography scanning areas for detection of glaucomatous damage. Am J Ophthalmol. 2005;139(1):39–43. [PubMed: 15652826]
- 68.
- Leung CK, Yung WH, Ng AC, et al. Evaluation of scanning resolution on retinal nerve fiber layer measurement using optical coherence tomography in normal and glaucomatous eyes. J Glaucoma. 2004;13(6):479–85. [PubMed: 15534473]
- 69.
- Mori S, Hangai M, Sakamoto A, et al. Spectral-domain optical coherence tomography measurement of macular volume for diagnosing glaucoma. J Glaucoma. 2010;19(8):528–34. [PubMed: 20164794]
- 70.
- Kim NR, Hong S, Kim JH, et al. Comparison of macular ganglion cell complex thickness by Fourier-Domain OCT in normal tension glaucoma and primary open-angle glaucoma. J Glaucoma. 2011 June 22 [Epub ahead of print] [PubMed: 21701394]
- 71.
- Oddone F, Centofanti M, Tanga L, et al. Influence of disc size on optic nerve head versus retinal nerve fiber layer assessment for diagnosing glaucoma. Ophthalmology. 2011;118(7):1340–7. [PubMed: 21474186]
- 72.
- Girkin CA, Liebmann J, Fingeret M, et al. The effects of race, optic disc area, age, and disease severity on the diagnostic performance of spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(9):6148–53. [PubMed: 21421879]
- 73.
- Leite MT, Rao HL, Zangwill LM, et al. Comparison of the diagnostic accuracies of the Spectralis, Cirrus, and RTVue optical coherence tomography devices in glaucoma. Ophthalmology. 2011;118(7):1334–9. [PMC free article: PMC3881436] [PubMed: 21377735]
- 74.
- Mansoori T, Viswanath K, Balakrishna N. Quantification of retinal nerve fiber layer thickness in normal eyes, eyes with ocular hypertension, and glaucomatous eyes with SD-OCT. Ophthalmic Surg Lasers Imaging. 2010;41(Suppl):S50–7. [PubMed: 21117601]
- 75.
- Horn FK, Mardin CY, Bendschneider D, et al. Frequency doubling technique perimetry and spectral domain optical coherence tomography in patients with early glaucoma. Eye. 2011;25(1):17–29. [PMC free article: PMC3144638] [PubMed: 21102494]
- 76.
- Shoji T, Sato H, Ishida M, et al. Assessment of glaucomatous changes in subjects with high myopia using spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(2):1098–102. [PubMed: 21051712]
- 77.
- Benitez-del-Castillo J, Martinez A, Regi T. Diagnostic capability of scanning laser polarimetry with and without enhanced corneal compensation and optical coherence tomography. Eur J Ophthalmol. 2011;21(3):228–36. [PubMed: 20872357]
- 78.
- Aptel F, Sayous R, Fortoul V, et al. Structure-function relationships using spectral-domain optical coherence tomography: comparison with scanning laser polarimetry. Am J Ophthalmol. 2010;150(6):825–33. [PubMed: 20851372]
- 79.
- Cho JW, Sung KR, Hong JT, et al. Detection of glaucoma by spectral domain-scanning laser ophthalmoscopy/optical coherence tomography (SD-SLO/OCT) and time domain optical coherence tomography. J Glaucoma. 2011;20(1):15–20. [PubMed: 20436370]
- 80.
- Reus NJ, Lemij HG, Garway-Heath DF, et al. Clinical assessment of stereoscopic optic disc photographs for glaucoma: the European Optic Disc Assessment Trial. Ophthalmology. 2010;117(4):717–23. [PubMed: 20045571]
- 81.
- Francis BA, Varma R, Vigen C, et al. Population and high-risk group screening for glaucoma: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2011;52(9):6257–64. [PMC free article: PMC3175989] [PubMed: 21245400]
- 82.
- Medeiros FA, Zangwill LM, Bowd C, et al. Comparison of scanning laser polarimetry using variable corneal compensation and retinal nerve fiber layer photography for detection of glaucoma. Arch Ophthalmol. 2004;122(5):698–704. [PubMed: 15136317]
- 83.
- Hong S, Ahn H, Ha SJ, et al. Early glaucoma detection using the Humphrey Matrix Perimeter, GDx VCC, Stratus OCT, and retinal nerve fiber layer photography. Ophthalmology. 2007;114(2):210–5. [PubMed: 17270671]
- 84.
- Chen HY, Huang ML, Tsai YY, et al. Comparing glaucomatous optic neuropathy in primary open angle and primary angle closure glaucoma eyes by scanning laser polarimetry-variable corneal compensation. J Glaucoma. 2008;17(2):105–10. [PubMed: 18344755]
- 85.
- Parikh RS, Parikh SR, Kumar RS, et al. Diagnostic capability of scanning laser polarimetry with variable cornea compensator in Indian patients with early primary open-angle glaucoma. Ophthalmology. 2008;115(7):1167–72. e1. [PubMed: 18061269]
- 86.
- Medeiros FA, Bowd C, Zangwill LM, et al. Detection of glaucoma using scanning laser polarimetry with enhanced corneal compensation. Invest Ophthalmol Vis Sci. 2007;48(7):3146–53. [PubMed: 17591884]
- 87.
- Mai TA, Reus NJ, Lemij HG. Diagnostic accuracy of scanning laser polarimetry with enhanced versus variable corneal compensation. Ophthalmology. 2007;114(11):1988–93. [PubMed: 17459481]
- 88.
- Kanamori A, Nagai-Kusuhara A, Escano MF, et al. Comparison of confocal scanning laser ophthalmoscopy, scanning laser polarimetry and optical coherence tomography to discriminate ocular hypertension and glaucoma at an early stage. Graefes Arch Clin Exp Ophthalmol. 2006;244(1):58–68. [PubMed: 16044326]
- 89.
- Da Pozzo S, Iacono P, Marchesan R, et al. Scanning laser polarimetry with variable corneal compensation and detection of glaucomatous optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2005;243(8):774–9. [PubMed: 15756574]
- 90.
- Reus NJ, Lemij HG. Diagnostic accuracy of the GDx VCC for glaucoma. Ophthalmology. 2004;111(10):1860–5. [PubMed: 15465547]
- 91.
- Salim S, Netland PA, Fung KH, et al. Assessment of the Student Sight Savers Program methods for glaucoma screening. Ophthalmic Epidemiol. 2009;16(4):238–42. [PubMed: 19874145]
- 92.
- de Pierre-Filho PT, Schimiti RB, de Vasconcellos JP, et al. Sensitivity and specificity of frequency-doubling technology, tendency-oriented perimetry, SITA Standard and SITA Fast perimetry in perimetrically inexperienced individuals. Acta Ophthalmol Scand. 2006;84(3):345–50. [PubMed: 16704696]
- 93.
- Tafreshi A, Sample PA, Liebmann JM, et al. Visual function-specific perimetry to identify glaucomatous visual loss using three different definitions of visual field abnormality. Invest Ophthalmol Vis Sci. 2009;50(3):1234–40. [PMC free article: PMC2848160] [PubMed: 18978349]
- 94.
- Racette L, Medeiros FA, Zangwill LM, et al. Diagnostic accuracy of the Matrix 24-2 and original N-30 frequency-doubling technology tests compared with standard automated perimetry. Invest Ophthalmol Vis Sci. 2008;49(3):954–60. [PMC free article: PMC2367320] [PubMed: 18326718]
- 95.
- Leeprechanon N, Giangiacomo A, Fontana H, et al. Frequency-doubling perimetry: comparison with standard automated perimetry to detect glaucoma. Am J Ophthalmol. 2007;143(2):263–71. [PubMed: 17178091]
- 96.
- Burgansky-Eliash Z, Wollstein G, Patel A, et al. Glaucoma detection with matrix and standard achromatic perimetry. Br J Ophthalmol. 2007;91(7):933–8. [PMC free article: PMC1955642] [PubMed: 17215267]
- 97.
- Sample PA, Medeiros FA, Racette L, et al. Identifying glaucomatous vision loss with visual-function-specific perimetry in the Diagnostic Innovations in Glaucoma Study. Invest Ophthalmol Vis Sci. 2006;47(8):3381–9. [PubMed: 16877406]
- 98.
- Salvetat ML, Zeppieri M, Tosoni C, et al. Non-conventional perimetric methods in the detection of early glaucomatous functional damage. Eye. 2010;24(5):835–42. [PubMed: 19696803]
- 99.
- Ng M, Racette L, Pascual JP, et al. Comparing the full-threshold and Swedish interactive thresholding algorithms for short-wavelength automated perimetry. Invest Ophthalmol Vis Sci. 2009;50(4):1726–33. [PMC free article: PMC2716113] [PubMed: 19074800]
- Executive Summary - Screening for Glaucoma: Comparative EffectivenessExecutive Summary - Screening for Glaucoma: Comparative Effectiveness
Your browsing activity is empty.
Activity recording is turned off.
See more...
