NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Chou R, Hartung D, Rahman B, et al. Treatment for Hepatitis C Virus Infection in Adults [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012 Nov. (Comparative Effectiveness Reviews, No. 76.)
Background
Hepatitis C virus (HCV) is the most common chronic bloodborne pathogen in the United States. HCV is primarily acquired by large or repeated percutaneous exposures to blood, with injection drug use being the strongest risk factor. Based on a national survey of households, approximately 1.6 percent of U.S. adults over 20 years of age have antibodies to HCV, indicating prior acute HCV infection.1 About 78 percent of patients with acute HCV infection develop chronic HCV infection, defined by the presence of persistent viremia.
Chronic HCV infection has a variable course, but it is a leading cause of complications from chronic liver disease, including cirrhosis, liver failure, and hepatocellular carcinoma (HCC). Chronic HCV infection is associated with an estimated 15,000 deaths each year in the United States,2 and it is the most common indication for liver transplantation among American adults, accounting for more than 30 percent of cases.3 The prevalence of chronic HCV infection is thought to have peaked in 2001 at 3.6 million people, and the yearly incidence has declined from more than 200,000 cases per year in the 1980s to around 16,000 cases in 2009.4, 5 However, complications related to chronic HCV infection, which frequently occur only after decades of infection, are expected to rise for another 10 to 13 years.4
The goal of antiviral treatment for chronic HCV infection is to prevent the long-term health complications associated with HCV infection, such as cirrhosis, hepatic decompensation, and liver cancer, but it is extremely difficult to design and carry out clinical trials long and large enough to provide direct evidence related to these outcomes. The sustained virologic response (SVR) rate, typically defined as the proportion of patients who experience a decline in HCV-RNA (hepatitis C virus ribonucleic acid) to undetectable levels 24 weeks following completion of antiviral treatment, is the standard marker of successful treatment in clinical trials because an SVR is strongly associated with the long-term absence of viremia.6, 7 Recent studies have evaluated the association between achieving an SVR and reductions in mortality, liver failure, and cancer.8, 9
In the early 2000s, the combination of “pegylated” interferon plus ribavirin became the standard antiviral treatment for HCV infection.10–12 Pegylation refers to the cross-linking of polyethylene glycol molecules to the interferon molecule, which delays renal clearance and thereby permits less frequent dosing (once weekly vs. three times a week with standard interferon).13 Dual therapy with pegylated interferon plus ribavirin is associated with higher SVR rates (about 55–60 percent overall) than either standard interferon plus ribavirin or pegylated interferon monotherapy. Currently, two pegylated interferons are available: pegylated interferon alfa-2a and pegylated interferon alfa-2b. Although previous reviews found insufficient evidence to determine whether combination therapy with pegylated interferon alfa-2a or pegylated interferon alfa-2b plus ribavirin is more effective,14, 15 more head-to-head trials directly comparing these two regimens are now available.16–19
A number of factors affect response to antiviral treatment. The two major pretreatment predictors of SVR are the viral genotype and the pretreatment viral load.11 In the United States, genotype 1 infection is found in around three-quarters of HCV-infected patients.20 HCV genotype 1 infection is associated with a substantially lower response to antiviral treatment than infection with genotypes 2 and 3, which are present in about 20 percent of HCV-infected patients. A pretreatment viral load of <600,000 international units per milliliter (IU/mL) is associated with higher likelihood of achieving an SVR.11 Other factors less consistently or less strongly associated with an increased likelihood of achieving an SVR include female sex, age less than 40 years, non-Black race, lower body weight (≤75 kg), absence of insulin resistance, elevated alanine aminotransferase levels, and absence of bridging fibrosis or cirrhosis on liver biopsy.11 Effects of race on the likelihood of achieving an SVR may be due in part to polymorphisms in the interleukin-28B (IL28B) gene.21, 22
An issue complicating antiviral treatment is the high rate of adverse effects observed with interferon-based therapy, including flulike symptoms, fatigue, and neuropsychiatric and hematologic adverse effects.23 Such adverse effects can be difficult to tolerate and can lead to premature discontinuation of therapy.
In 2011, the U.S. Food and Drug Administration (FDA) approved the first direct acting antiviral agents, boceprevir (trade name Victrelis™) and telaprevir (trade name Incivek®), for treatment of chronic HCV genotype 1 infection.24, 25 Both drugs are classified as nonstructural 3/4A protease inhibitors, with a potential advantage of shorter duration of therapy (24 to 28 weeks) compared with standard dual therapy with pegylated interferon (alfa-2a or 2b) plus ribavirin for genotype 1 infection (48 weeks).26–28 Either drug is administered in combination with pegylated interferon (alfa-2a or 2b) plus ribavirin.
Understanding the comparative benefits and harms of the various antiviral regimens is critical for making informed treatment decisions in patients with chronic HCV infection, particularly given the availability of new treatment options. This review assesses the comparative effectiveness of antiviral treatments in adults with chronic HCV infection who have not received previous antiviral drug treatment. In addition to assessing the comparative effectiveness of different drug regimens, the review evaluates the effects of different medication doses, durations of therapy, and dosing strategies (such as weight-based or response-guided vs. fixed treatment). To help with individualized clinical decisionmaking regarding antiviral therapy for chronic HCV infection, the review also evaluates how comparative effectiveness varies depending on HCV genotype, viral load, and other demographic and clinical characteristics. Given the need to understand the effects of treatment in people with HCV infection identified by screening in order to assess the potential benefits and harms of screening, this review will be used, together with a separate review on HCV screening,29 by the U.S. Preventive Services Task Force to update its HCV screening recommendations.
Objectives
The following Key Questions are the focus of our report:
Key Question 1
- What is the comparative effectiveness of antiviral treatment in improving health outcomes in patients with HCV infection?
- How does the comparative effectiveness of antiviral treatment for health outcomes vary according to patient subgroup characteristics, including but not limited to HCV genotype, age, race, sex, stage of disease, or genetic markers?
Key Question 2
- What is the comparative effectiveness of antiviral treatments on intermediate outcomes, such as the rate of SVR or histologic changes in the liver?
- How does the comparative effectiveness of antiviral treatment for intermediate outcomes vary according to patient subgroup characteristics, including but not limited to HCV genotype, age, race, sex, stage of disease, or genetic markers?
Key Question 3
- What are the comparative harms associated with antiviral treatments?
- Do these harms differ according to patient subgroup characteristics, including HCV genotype, age, race, sex, stage of disease, or genetic markers?
Key Question 4
Have improvements in intermediate outcomes (SVR, histologic changes) been shown to reduce the risk or rates of adverse health outcomes from HCV infection?
Analytic Framework
The analytic framework that guided this report is shown in Figure A. The numbers in the analytic framework indicate the Key Questions listed above. The population was patients with chronic HCV infection who were receiving antiviral therapy. The interventions were dual therapy with pegylated interferon (alfa-2a or alfa-2b) plus ribavirin, or triple therapy with pegylated interferon (alfa-2a or alfa-2b) plus ribavirin plus a protease inhibitor approved by the FDA (either boceprevir or telaprevir). Comparisons were between different regimens, as well as between regimens including the same drugs administered at different doses or for different durations. Intermediate outcomes were sustained virologic response and hepatic histological improvement. Final outcomes were morbidity and mortality from HCV infection (including hepatic cirrhosis, HCC, and liver transplantation rates) and quality of life, as well as harms of antiviral therapies (including flulike symptoms, hematologic effects, rash, and psychiatric effects).
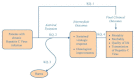
Figure A
Analytic framework for treatment of hepatitis C infection in adults. KQ = Key Question
Methods
Input From Stakeholders
The topic of treatment for HCV infection was nominated for a comparative effectiveness review (CER) in a public process. The Key Questions were proposed in the public nomination process and developed by investigators from the Evidence-based Practice Center (EPC) with contributions from expert Key Informants (KI), who helped refine Key Questions, identify important methodological and clinical issues, and define parameters for the review of evidence. The revised Key Questions were then posted to a public Web site for comment. The Agency for Healthcare Research and Quality (AHRQ) and the EPC agreed on the final Key Questions after reviewing the public comments and receiving additional advice from a Technical Expert Panel (TEP) convened for this report. We then drafted a protocol for this CER, which the TEP reviewed. Access it from the AHRQ Web site, where it was posted in November 2011: (www.effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productid=855).
A multidisciplinary group of clinicians, researchers, and patient advocates with expertise in hepatitis C treatment and research were selected to serve as the TEP members to provide high-level content and methodological expertise throughout the development of the review. Prior to participation in this report, the TEP members disclosed all financial or other conflicts of interest. The AHRQ Task Order Officer and the authors reviewed all of these disclosures and determined the panel members had no significant conflicts of interest that precluded participation. KIs and TEP members had expertise in hepatology, epidemiology, screening, and primary care. TEP members and other experts were invited to provide external peer review of the draft report.
Search Strategy and Study Selection
To identify articles relevant to each Key Question, a research librarian searched the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, and Ovid MEDLINE® from 1947 to April 2011 (see Appendix A for the search strategies), and a final updated search was conducted in August 2012. The search strategies were peer reviewed by another research librarian and revised prior to finalization. Unpublished trials were sought by searching clinical trial registries (ClinicalTrials.gov, Current Controlled Trials, Clinical Trial Results, WHO Trial Registries) and grants databases (NIHRePORTER, HSRProj, and AHRQ GOLD). Scientific Information Packets on unpublished and published trials were solicited from manufacturers of included antiviral drugs through the Scientific Resource Center. We also hand-searched the reference lists of relevant studies. Searches were updated before the report was finalized to identify relevant new publications.
Studies were selected according to criteria developed for inclusion and exclusion. The selection criteria were based on the Key Questions and the populations, interventions, comparators, outcomes, timing, and setting (PICOTS) approach. Papers were selected for full review if they were about chronic HCV infection, were relevant to Key Questions in the analytic framework, and met the predefined inclusion criteria. To evaluate the potential effects of publication bias, we included trials published only as conference abstracts of sensitivity analyses. We restricted inclusion to English language articles. Studies of nonhuman subjects were also excluded, and studies had to include original data.
Abstracts and full-text articles were dual reviewed for inclusion and exclusion for each Key Question. Full-text articles were obtained for all studies identified as potentially meeting inclusion criteria. Two investigators independently reviewed all full-text articles for final inclusion or exclusion, and discrepancies were resolved through discussion and consensus, with a third investigator making the final decision if necessary.
Data Extraction and Quality Assessment
We assessed the quality of each study based on predefined criteria (Appendix E). We adapted criteria from methods proposed by Downs and Black (observational studies),30 the USPSTF,31 and the Quality Assessment of Diagnostic Accuracy Studies-2 Group.32 The criteria used are consistent with the approach recommended by AHRQ in the Methods Guide for Effectiveness and Comparative Effectiveness Reviews (Methods Guide).33 We used the term “quality” rather than the alternate term “risk of bias.” Although both refer to internal validity, “quality” may be more familiar to most users and has potential advantages in terms of readability.
We rated the quality of each randomized trial based on the methods used for randomization, allocation concealment, and blinding; the similarity of compared groups at baseline; maintenance of comparable groups; adequate reporting of dropouts, attrition, crossover, adherence, and contamination; loss to followup; the use of intent-to-treat analysis; and ascertainment of outcomes.31
We rated the quality of each cohort study based on whether it used nonbiased selection methods to create an inception cohort; whether it evaluated comparable groups; whether rates of loss to followup were reported and acceptable; whether it used accurate methods for ascertaining exposures, potential confounders, and outcomes; and whether it performed appropriate statistical analyses of potential confounders.31
Following assessment of individual quality criteria, individual studies were rated good, fair, or poor quality, as defined below.33
Good-quality studies are considered likely to be valid. Good-quality studies clearly describe the population, setting, interventions, and comparison groups; use a valid method for allocation of patients to interventions; clearly report dropouts and have low dropout rates; use appropriate methods for preventing bias; and appropriately measure outcomes and fully report results.
Fair-quality studies have some methodological deficiencies but no flaw or combination of flaws judged likely to cause major bias. The study may be missing information, making it difficult to assess its methods or assess limitations and potential problems. The fair-quality category is broad, and studies with this rating vary in their strengths and weaknesses—the results of some fair-quality studies are likely to be valid, while others are only probably valid.
Poor-quality studies have significant flaws that may invalidate the results. They have a serious or fatal flaw in design, analysis, or reporting; large amounts of missing information; or discrepancies in reporting. The results of these studies are judged to be at least as likely to reflect flaws in the study design as true effects of the interventions under investigation. We did not exclude studies rated poor quality a priori, but they were considered to be the least reliable studies when synthesizing the evidence, particularly when discrepancies between studies were present.
We recorded factors important for understanding the applicability of studies, such as whether the publication adequately described the study population, how similar patients were to populations likely to be targeted by screening, whether differences in outcomes were clinically (as well as statistically) significant, and whether the interventions and tests evaluated were reasonably representative of standard practice.34 We also recorded the funding source and role of the sponsor. We did not assign a rating of applicability (such as high or low) because applicability may differ based on the user of this report.
Data Synthesis and Rating the Strength of the Body of Evidence
We performed meta-analysis of trials that evaluated similar populations, interventions, comparisons, and outcomes to estimate pooled relative risks.35 When present, statistical heterogeneity was explored through subgroup and sensitivity analyses, as well as qualitatively. Subgroup analyses were performed in groups stratified by HCV genotype as well as by race, age, body weight, viral load, stage/severity of disease, and IL-28b status when these data were available. We performed sensitivity analysis by excluding poor-quality studies and outlier trials, and by including results from studies published only as abstracts to evaluate the stability of estimates and conclusions. We did not perform meta-analyses for Key Question 4 because all studies were observational and had important methodologic shortcomings. These studies were synthesized qualitatively.
We rated the strength of evidence for each Key Question using the four categories recommended in the AHRQ Methods Guide.33 We synthesized the overall quality of each body of evidence based on the type and quality of studies (graded good, fair, or poor); the precision of the estimate of effect based on the number and size of studies and confidence intervals for the estimates (graded high, moderate, or low); the consistency of results between studies (graded high, moderate, or low); and the directness of the evidence linking the intervention and health outcomes (graded direct or indirect). We did not downgrade a body of evidence for directness that evaluated an intermediate outcome if the intermediate outcome was the specific focus of the Key Question. We were not able to formally assess for publication bias due to small numbers of studies, methodological shortcomings, or differences across studies in designs, measured outcomes, and other factors.
We graded the strength of evidence for each comparison and outcome by using the four categories recommended in the AHRQ Methods Guide:33 A “high” grade indicates high confidence that the evidence reflects the true effect and that further research is very unlikely to change our confidence in the estimate of effect and will not change the estimate. A “moderate” grade indicates moderate confidence that the evidence reflects the true effect and that further research may change our confidence in the estimate of effect and may change the estimate. A “low” grade indicates low confidence that the evidence reflects the true effect and that further research is likely to change the confidence in the estimate of effect and is likely to change the estimate. An “insufficient” grade indicates evidence either is unavailable or is too limited to permit any conclusion.
Results
The search and selection of articles are summarized in the study flow diagram (Figure B). Of the 1,096 citations identified at the title and abstract level in the original search, 215 articles met inclusion criteria and were selected for further review of the full text. From updated searches and peer reviewer suggested citations, an additional 2,352 citations were identified, and 164 of these met inclusion criteria and were selected for full-text review. Of the 379 articles reviewed at the full-text level, a total of 90 studies met inclusion criteria.
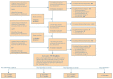
Figure B
Study flow diagram: Treatment for hepatitis C virus infection in adults.
No study evaluated comparative effectiveness of current antiviral regimens on long-term clinical outcomes such as mortality, complications of chronic HCV infection, or quality of life.
Dual Therapy Regimens with Pegylated Interferon Plus Ribavirin
In trials of treatment-naïve patients, dual therapy with pegylated interferon alfa-2b plus ribavirin was associated with a slightly lower likelihood of achieving an SVR than dual therapy with pegylated interferon alfa-2a plus ribavirin, with a difference in absolute SVR rates of about 8 percentage points.16–19, 36–38 In patients with genotype 2 or 3 infection, dual therapy for 12 to 16 weeks appears to be associated with a lower likelihood of SVR, compared with dual therapy for 24 weeks, with no differences between 24 weeks and longer courses of therapy.39–44 In trials comparing different doses of dual therapy with pegylated interferon plus ribavirin, lower doses of pegylated interferon alfa-2b were less effective than standard doses,41, 45–49 and limited evidence found no clear differential effects of ribavirin dosing.39, 50
There were no clear differences in estimates of relative effectiveness between dual therapy with pegylated interferon alfa-2a plus ribavirin versus dual therapy with pegylated interferon alfa-2b plus ribavirin in patient subgroups defined by demographic or clinical characteristics, although absolute response rates were lower in older patients, Black patients, patients with high viral load, patients with more advanced fibrosis or cirrhosis, and patients with genotype 1 infection.16, 17, 19, 51
Differences in harms between dual therapy with pegylated interferon alfa-2a plus ribavirin versus pegylated interferon alfa-2b plus ribavirin were relatively small, with no differences in withdrawals due to adverse events, although dual therapy with pegylated interferon alfa-2b was associated with a lower risk of serious adverse events.16–19, 38, 52
Triple Therapy Regimens With Pegylated Interferon, Ribavirin, and Either Boceprevir or Telaprevir
Trials of antiviral regimens including either boceprevir or telaprevir have been primarily conducted in patients with genotype 1 infection. Triple antiviral regimens (pegylated interferon alfa-2a or alfa-2b, ribavirin, and boceprevir or telaprevir) were associated with a substantially increased likelihood of achieving an SVR than dual therapy with pegylated interferon alfa-2a or alfa-2b plus ribavirin).26–28, 53–57
Two trials found triple therapy with boceprevir for 48 weeks (dual therapy with pegylated interferon alfa-2b plus ribavirin for 4 weeks followed by 44 weeks of triple therapy with the addition of boceprevir) was associated with a higher likelihood of SVR than dual therapy with pegylated interferon alfa-2b plus ribavirin for 48 weeks (pooled relative risk [RR] 1.81, 95% confidence interval [CI] 1.58 to 2.06, I2=0.0%) with an absolute increase in SVR rate of 31 percentage points (95% CI 23 to 39).26, 28
Three trials found triple therapy with telaprevir for 24 weeks (pegylated interferon alfa-2a, ribavirin, and telaprevir triple therapy for 12 weeks followed by 12 weeks of pegylated interferon alfa-2a plus ribavirin without telaprevir) was associated with a higher likelihood of SVR than dual therapy with pegylated interferon alfa-2a plus ribavirin for 48 weeks (pooled RR 1.48, 95% CI 1.26 to 1.75, I2=0.0%), with an absolute increase in SVR rate of 22 percentage points (95% CI 13 to 31).27, 53, 55 One trial found response-guided telaprevir triple therapy (8 or 12 weeks of pegylated interferon alfa-2a, ribavirin, and telaprevir followed by 12 or 36 weeks of response-guided dual therapy with pegylated interferon alfa-2a plus ribavirin) was associated with a higher likelihood of SVR than dual therapy with pegylated interferon alfa-2a plus ribavirin for 48 weeks (RR 1.6, 95% CI 1.4 to 1.9), with an absolute increase in SVR rate of 25–31 percentage points.54
Relative estimates of the effects of triple therapy with either boceprevir or telaprevir, compared with dual therapy, were similar across subgroups, except in patients with low viral load, in whom triple therapy was no more effective than dual therapy in achieving an SVR. Triple therapy with boceprevir was associated with increased risk of hematological adverse events and triple therapy with telaprevir with increased risk of anemia and rash (including severe rash) than dual therapy; adverse events were generally self-limited with discontinuation of therapy.26, 28 All antiviral regimens were associated with a high incidence of flulike symptoms, with small or no clear differences in risk.
Sustained Virologic Response After Antiviral Therapy and Clinical Outcomes
A large cohort study that was well controlled for confounders found that patients with an SVR after antiviral therapy had a lower risk of all-cause mortality than patients with no SVR (adjusted hazard ratio estimates 0.51 to 0.71).8 Eighteen other cohort studies also found SVR associated with reduced risk of all-cause mortality, liver-related mortality, and other hepatic complications rather than no SVR, but had more methodological shortcomings.9, 58–74 Ten of the studies were conducted in Asian countries and might not be directly applicable to U.S. populations.
Discussion
Key Findings and Strength of Evidence
The evidence reviewed in this study is summarized in Table A. The specific domain scores used to determine the overall strength of evidence for each body of evidence are shown in Appendix G. We identified no studies that evaluated comparative effectiveness of current antiviral regimens on long-term clinical outcomes such as mortality, complications of chronic HCV infection, or quality of life. Such trials would be difficult to design and carry out due to the long time required for complications of chronic HCV infection to develop in most patients.
Table A
Summary of evidence on comparative effectiveness of treatment for hepatitis C.
Dual Therapy Regimens With Pegylated Interferon and Ribavirin
In lieu of direct evidence on long-term clinical outcomes, SVR rates are the primary outcome to assess comparative benefits of different antiviral regimens. In trials of treatment-naïve patients, the likelihood of achieving an SVR was slightly lower with dual therapy with pegylated interferon alfa-2b plus ribavirin compared with dual therapy with pegylated interferon alfa-2a plus ribavirin (pooled RR 0.87, 95% CI 0.80 to 0.95; I2=27.4%), with a difference in absolute SVR rates of about 8 percentage points. Although the largest study, the Individualized Dosing Efficacy vs. Flat Dosing to Assess Optimal Pegylated Interferon Therapy (IDEAL) trial, found no difference in SVR rates for dual therapy with pegylated interferon alfa-2a plus ribavirin compared with dual therapy with pegylated interferon alfa-2b plus ribavirin, excluding the IDEAL trial from pooled analyses, resulted in similar effect estimates.18 Although there was no difference between types of dual therapy regimens in risk of withdrawals due to adverse events, dual therapy with pegylated interferon alfa-2b plus ribavirin was associated with a lower risk of serious adverse events than dual therapy with pegylated interferon alfa-2a plus ribavirin (pooled RR 0.76, 95% CI 0.71 to 0.88, I2=0.0%), suggesting a potential tradeoff between greater benefits and greater harms. However, serious adverse events were only reported in two trials,18, 19 and the rate of serious adverse events was relatively low (about 4 percent overall in IDEAL), with an absolute difference of about 1 percent, and adverse events with antiviral treatments generally resolve following discontinuation of therapy. Trials found no clear difference in estimates of relative effectiveness of dual therapy with pegylated interferon alfa-2a plus ribavirin compared with dual therapy with pegylated interferon alfa-2b plus ribavirin in patient subgroups stratified by age, sex, race, viral load, fibrosis stage, and genotype, although absolute response rates were lower in older patients, Black patients, patients with high viral load, patients with more advanced fibrosis or cirrhosis, and patients with genotype 1 infection.16–19, 51 SVR rates ranged from 24 to 42 percent lower in patients with genotype 1 infection compared with patients with genotype 2 or 3.
In patients with genotype 2 or 3 infection, dual therapy for 12 to 16 weeks appears to be associated with a lower likelihood of SVR compared with dual therapy for 24 weeks, with no differences between 24 weeks and longer courses of therapy.39–44 Standard doses of pegylated interferon alfa-2b were more effective than lower doses (no trials compared different doses of pegylated interferon alfa-2a).41, 45–49 Although trials comparing different ribavirin doses found no clear differences, they evaluated different dose comparisons, precluding firm conclusions.39, 50, 75, 76
Triple Therapy Regimens With Pegylated Interferon, Ribavirin, and Either Boceprevir or Telaprevir
Trials of triple therapy regimens with the protease inhibitors boceprevir or telaprevir (both approved by the FDA in 2011) in treatment-naïve patients with genotype 1 infection found each associated with substantially higher SVR rates than standard dual therapy without a protease inhibitor. SVR rates with triple therapy were similar to the 70–80 percent observed with dual therapy in patients with genotype 2 or 3 infection.23, 26–28, 53–57, 77 Trials that evaluated the telaprevir regimen recommended by the FDA (12 weeks of triple therapy with telaprevir followed by response-guided duration of 12 or 36 weeks of dual therapy) reported SVR rates of 75–80 percent.54, 56 Trials that evaluated the boceprevir regimen recommended by the FDA for antiviral-naïve patients with cirrhosis (4 weeks of dual therapy lead-in followed by 44 weeks of triple therapy with boceprevir) reported SVR rates of 66–75 percent.26, 28 Trials that evaluated other regimens in antiviral naïve patients, including fixed duration telaprevir regimens, shorter fixed duration triple therapy boceprevir therapy, and boceprevir without dual therapy lead-in, reported similar or lower SVR rates.
As with the head-to-head trials of dual therapy with pegylated interferon alfa-2a plus ribavirin compared with pegylated interferon alfa-2b plus ribavirin, RR estimates for triple, compared with dual, therapy were similar (or there were no clear differences) in patient subgroups based on age, sex, or race, although absolute SVR rates were lower in older patients and Black patients. In two trials, triple therapy with boceprevir was no more effective than dual therapy in the subgroup of patients with lower HCV-RNA viral load (<600,000 or <800,000 IU/mL),26, 28 but two trials of triple therapy with telaprevir were inconsistent in showing differential effects depending on baseline viral load.54, 55 There was insufficient evidence to evaluate relative effectiveness of triple, compared with dual, therapy based on fibrosis stage.
In addition to a higher likelihood of SVR, another advantage of triple therapy regimens in patients with genotype 1 infection is the potential for a shorter duration of treatment (24 or 28 weeks in patients with early virologic response, compared with the standard 48 weeks of dual therapy with pegylated interferon plus ribavirin). Shorter courses of treatment would probably be appealing to patients, given the frequency of bothersome flulike symptoms associated with interferon-based therapy. On the other hand, triple therapy regimens were associated with increased risk of certain harms, in particular hematological adverse events (neutropenia, anemia, and thrombocytopenia) with boceprevir, and anemia and rash (including severe rash in up to about 10 percent of patients, which could result in treatment discontinuation) with telaprevir. However, there was no clear increase in risk of serious adverse events or overall withdrawal due to adverse events with use of protease inhibitors, and the adverse events appear to be self-limited following drug discontinuation.
Sustained Virologic Response After Antiviral Therapy, and Clinical Outcomes
The strongest evidence on the association between an SVR after antiviral therapy and improved clinical outcomes is a large U.S. Department of Veterans Affairs (VA) cohort study (n=16,864) that adjusted for many confounders and found decreased risk of all-cause mortality compared with no SVR across patient groups stratified by genotype (adjusted hazard ratio [HR] 0.71 [0.60–0.86], 0.62 [0.44–0.87] and 0.51 [0.35–0.75] for genotypes 1, 2, and 3, respectively).8 Despite controlling for important confounders, the possibility of residual confounding is suggested by the very rapid separation of mortality curves for people with an SVR versus those without an SVR, which was observed at 3 months after assessment for SVR. This is more rapid than expected given the typically prolonged natural history of HCV infection. Therefore, estimates of effects of SVR on clinical outcomes from this study may be exaggerated, although it is not possible to determine to what degree. Eighteen other cohort studies also found an SVR after antiviral therapy associated with decreased risk of all-cause mortality and complications of chronic HCV infection, including studies specifically of patients with baseline cirrhosis, but had more methodological shortcomings. In addition, 10 of the 19 studies were conducted in Asia, where the incidence of HCC in patients with chronic HCV infection is higher than in the United States,78 potentially limiting their generalizability. Other studies found an SVR after antiviral therapy associated with better scores on measures of quality of life than with no SVR, but those studies focused on short-term outcomes and typically did not adjust for confounders or blind patients to SVR status when assessing outcomes.
Findings in Relationship to What Is Already Known
Our findings regarding the comparative effectiveness of dual therapy with pegylated interferon alfa-2b plus ribavirin compared with dual therapy with pegylated interferon alfa-2a plus ribavirin are consistent with recent systematic reviews that also found the former associated with a lower likelihood of SVR.14, 79 Our findings of no clear difference in comparative effectiveness between 12 to 16 weeks compared with 24 weeks of response-guided dual therapy with pegylated interferon plus ribavirin in hepatitis C genotype 2 or 3 infection with rapid virologic response are discordant with a recent systematic review, which found a shorter duration of treatment associated with a lower likelihood of achieving an SVR.80 The discrepancy may be explained by the inclusion in the other systematic review of a study that we excluded because it evaluated a nonstandard dose of pegylated interferon,81 as well as its inclusion of subgroup analyses from trials of patients randomized to different fixed durations of therapy prior to assessment of rapid virologic response,40, 42, 43 which we considered separately because they did not represent randomized comparisons of response-guided treatment.
Because telaprevir and boceprevir are so new, we are unaware of other published systematic reviews on the comparative benefits and harms of regimens including these drugs, compared with standard dual therapy. Our findings on the association between achieving an SVR and reduced risk of mortality or complications associated with chronic HCV infection are consistent with a recent review that used some systematic methods.82
Applicability
The trials included in this review generally met criteria for efficacy studies based on the exclusion of patients with common comorbidities (such as serious psychiatric conditions or recent or ongoing substance abuse). In addition, the trials may have overestimated efficacy compared with what would be seen in typical practice due to improved adherence as a result of closer followup, effects of trial participation, selection of patients, or other factors. A separate review funded by AHRQ will be focusing on issues related to the screening for HCV infection in adults. 29
The severity of baseline liver disease in the patients enrolled in the trials suggests a broad range of patients were enrolled. In trials of triple therapy with boceprevir or telaprevir, the proportion of patients with cirrhosis at enrollment ranged from <1 to 11 percent.26–28, 53, 54, 56, 57 Trials that reported the proportion of patients with minimal or no fibrosis reported rates of 27–39 percent.27, 53, 54, 56, 57
Evidence to evaluate potential differences in comparative benefits or harms in patient subgroups based on age, sex, race, and other clinical factors was relatively limited, precluding strong conclusions in these specific subgroups. The strongest evidence on the association between an SVR versus no SVR after antiviral therapy and reduced mortality comes from a study performed in a VA population, which might limit generalizability to other settings.8 As described above, studies conducted in Asia on the association between an SVR after antiviral therapy and risk of clinical outcomes may be of limited applicability to U.S. populations because of a higher incidence of HCC in Asian patients with chronic HCV infection.78 However, the incidence of HCC is increasing in the United States in HCV-infected people,83 which may attenuate such concerns regarding applicability.78
The results of this CER are not applicable to populations excluded from the review, including patients previously treated with antiviral therapies and excluded populations such as patients with Human immunodeficiency virus (HIV) coinfection, post-transplant patients, or hemodialysis patients. Antiviral therapy is not recommended in patients following kidney transplant, and ribavirin is not recommended in those with more severe (stage 3 to 5) kidney disease since it is cleared via renal function and associated with increased risk of hemolytic anemia in this setting.84 Such patients were typically excluded from randomized trials of antiviral treatment.
Implications for Clinical and Policy Decisionmaking
Our review has potential implications for clinical and policy decisionmaking. For patients with genotype 1 infection, triple therapy regimens with pegylated interferon alfa-2a or alfa-2b, ribavirin, and telaprevir or boceprevir may be considered an alternative to dual therapy with pegylated interferon alfa-2a or alfa-2b plus ribavirin as standard treatment due to substantially superior efficacy for achieving SVR compared with dual therapy with pegylated interferon alfa-2a or alfa-2b, as well as a shorter duration of treatment. Factors that may affect decisions to use regimens with boceprevir or telaprevir include cost and specific harms associated with use of these drugs (such as hematologic adverse events with boceprevir and anemia and rash with telaprevir). Dual therapy with pegylated interferon alfa-2a plus ribavirin appears to be associated with a higher likelihood of achieving SVR compared with dual therapy with pegylated interferon alfa-2b plus ribavirin, but absolute differences were relatively small. Therefore, decisions about which pegylated interferon to use may be affected by other considerations, such as cost, patient preferences, or other factors. For genotype 2 or 3 infection, standard doses and duration (24 weeks) of pegylated interferon as part of dual therapy are more effective than shorter regimens or lower doses, lending support to dosing guidance from the FDA and clinical practice guidelines.11, 85, 86 Evidence on differential effects of ribavirin dose are too limited to draw strong conclusions about optimal dosing of this component of antiviral regimens, although differences appeared relatively small.
The findings that absolute SVR rates are lower in certain subgroups (such as older patients, Black patients, patients with worse baseline fibrosis, and patients with high viral load) can be used to guide individualized decisionmaking. Patients who are less likely to achieve an SVR may make different informed decisions about therapy compared with those more likely to achieve an SVR, given the adverse effects associated with treatment.
The findings of the review are also relevant to screening recommendations, which are based in part on the effectiveness of treatments in people found through screening to have HCV infection. Important new evidence that may affect assessments regarding potential benefits of screening include stronger evidence on the link between achieving an SVR and improvement in clinical outcomes, as well as evidence showing substantially higher SVR rates with newer triple therapy regimens with boceprevir or telaprevir in patients with genotype 1 infection, the predominant type of HCV infection in the United States.
Limitations of the Comparative Effectiveness Review Process
Our review had some potential limitations. We excluded non–English-language articles, which could result in language bias, although a recent systematic review found little empirical evidence that exclusion of non–English-language articles leads to biased estimates for noncomplementary or alternative medicine interventions.87
We did not formally assess for publication bias with funnel plots due to small numbers (<10) of studies for all comparisons. Small numbers of studies can make interpretation of funnel plots unreliable, and experts suggest 10 studies as the minimum number of studies to perform them.88 We included some studies that were published only as abstracts and found their inclusion or exclusion from analyses did not change conclusions. In addition, we searched trial registries and solicited drug manufacturers for additional unpublished trials and identified none.
Another potential limitation is that we included cohort studies to evaluate the association between SVR and either mortality or hepatic complications associated with chronic HCV infection. Such studies are susceptible to confounding if factors associated with SVR (such as age, race, viral load, or fibrosis stage) are also associated with these outcomes. Therefore, we only included studies that reported adjusted risk estimates, and we evaluated how well studies addressed key potential confounders as part of our quality assessment. Nonetheless, residual confounding is a possibility, even in cohort studies that adjust for potential confounding.
Limitations of the Evidence Base
We identified several important limitations of the evidence base. First, studies assessing important long-term clinical outcomes associated with current antiviral treatments for chronic HCV infection are not available. In the case of antiviral regimens involving newly approved antiviral drugs, such studies are not possible yet because of the extended followup required to adequately evaluate effects on clinical outcomes. Second, no trials directly compared regimens with boceprevir with regimens with telaprevir. Given the increased efficacy of these regimens for genotype 1 infection, trials directly comparing their effects would be helpful for guiding health care providers’ treatment choices between these drugs. Third, few trials have evaluated the regimens approved specifically by the FDA for these drugs, limiting confidence in conclusions regarding estimates of benefits and harms for the regimens likely to be used in clinical practice. Fourth, few methodologically rigorous studies conducted in settings applicable to U.S. populations evaluated the association between achieving an SVR and improvements in clinical outcomes. Such studies would be very helpful for confirming the results of the recent large, well-conducted VA cohort study showing an association between achieving an SVR and reduced mortality risk.8
Future Research
Evaluating the comparative effectiveness of current antiviral regimens on clinical outcomes in randomized trials or cohort studies is a challenge due to the long lead time and large sample sizes necessary to adequately assess these outcomes. This might be more feasible if the studies were to focus on populations at higher risk for complications from chronic HCV infection (e.g., patients with baseline cirrhosis, high viral load, or other risk factors for progression).
For all trials of antiviral treatments, studies that enroll broader populations with medical and psychological comorbidities, as frequently encountered in clinical practice, are needed to better understand comparative effectiveness, rather than just comparative efficacy. Studies designed using an effectiveness paradigm would also be helpful for understanding real-world outcomes of antiviral regimens, including effects related to the poorer treatment adherence than expected from efficacy trials.
Trials directly comparing triple therapy with telaprevir compared with triple therapy with boceprevir would be very helpful for understanding comparative effectiveness of these two protease inhibitors. In addition, trials evaluating the boceprevir regimen recommended by the FDA in antiviral-naïve patients without baseline cirrhosis are needed to verify that results from studies of previously treated patients were appropriately generalized. Prolonged followup of patients exposed to telaprevir and boceprevir is needed to understand the long-term harms associated with these medications. A number of other protease inhibitors and other newer drugs for treatment of hepatitis C virus infection are currently in active development, and further studies with new drugs and drug regimens are expected, including regimens without interferon.89
It is critical that future studies that evaluate clinical outcomes in patients with an SVR versus no SVR after antiviral therapy adequately control for other factors that influence clinical outcomes in chronic HCV infection. Studies on effects of achieving an SVR on long-term quality of life would be very helpful for understanding other potential clinical benefits of antiviral therapy, but a significant challenge is whether it is possible to ethically blind patients to virologic status, which may have an important effect on assessments of quality of life.
References
- 1.
- Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14. [PubMed: 16702586]
- 2.
- Ly KN, Xing J, Klevens RM, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271–8. [PubMed: 22351712]
- 3.
- Kim WR. The burden of hepatitis C in the United States. Hepatology. 2002;36(5 suppl 1):S30–S4. [PubMed: 12407574]
- 4.
- Davis GL, Alter MJ, El-Serag H, et al. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterol. 2010 Feb;138(2):513–21. [PubMed: 19861128]
- 5.
- National Center for HIV/AIDS VH, STD & TB Prevention. Disease burden from viral hepatitis A, B, and C in the United States. Centers for Disease Control and Prevention; 2011. [Accessed on 6/29/12]. www
.cdc.gov/hepatitis /PDFs/disease_burden.pdf. - 6.
- Swain M, Lai MY, Shiffman ML, et al. Durability of sustained virological response (SVR) after treatment with peginterferon alfa-2A (40KD) (PEGASYS) alone or in combination with ribavirin (COPEGUS): results of an ongoing long-term follow-up study. Hepatology (Baltimore, MD). 2004;40(4 Suppl 1):400A–1A.
- 7.
- Swain MG, Lai MY, Shiffman ML, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterol. 2010 Nov;139(5):1593–601. [PubMed: 20637202]
- 8.
- Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509–16. [PubMed: 21397729]
- 9.
- Morgan TR, Ghany MG, Kim H-Y, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010 Sep;52(3):833–44. [PMC free article: PMC2932862] [PubMed: 20564351]
- 10.
- Anonymous. National Institutes of Health consensus development conference statement: management of hepatitis C: 2002--June 10–12, 2002. Hepatology. 2002;36(5 (Suppl 1)):S3–S20. [PubMed: 12407572]
- 11.
- Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–74. [PMC free article: PMC7477893] [PubMed: 19330875]
- 12.
- Strader DB, Wright T, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39(4):1147–71. [PubMed: 15057920]
- 13.
- Foster GR. Review article: pegylated interferons: chemical and clinical differences. Aliment Pharmacol Ther. 2004 Oct 15;20(8):825–30. [PubMed: 15479353]
- 14.
- Awad T, Thorlund K, Hauser G, et al. Peginterferon alpha-2a is associated with higher sustained virological response than peginterferon alfa-2b in chronic hepatitis C: Systematic review of randomized trials. Hepatology. 2010;51(4):1176–84. [PubMed: 20187106]
- 15.
- Chou RCS, Chan BKS. Drug Class Review on Pegylated Interferons for Chronic Hepatitis C Infection. Portland, OR: Oregon Health & Science University; 2007. www
.ohsu.edu/drugeffectiveness /reports/final.cfm. - 16.
- Ascione A, De Luca M, Tartaglione MT, et al. Peginterferon alfa-2a plus ribavirin is more effective than peginterferon alfa-2b plus ribavirin for treating chronic hepatitis C virus infection. Gastroenterol. 2010 Jan;138(1):116–22. [PubMed: 19852964]
- 17.
- Escudero A, Rodriguez F, Serra MA, et al. Pegylated alpha-interferon-2a plus ribavirin compared with pegylated alpha-interferon-2b plus ribavirin for initial treatment of chronic hepatitis C virus: prospective, non-randomized study. J Gastroenterol Hepatol. 2008 Jun;23(6):861–6. [PubMed: 18422960]
- 18.
- McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009 Aug 6;361(6):580–93. [Erratum appears in N Engl J Med. 2009 Sep 3;361(10):1027] [PubMed: 19625712]
- 19.
- Rumi MG, Aghemo A, Prati GM, et al. Randomized study of peginterferon-alpha2a plus ribavirin vs peginterferon-alpha2b plus ribavirin in chronic hepatitis C. Gastroenterol. 2010 Jan;138(1):108–15. [PubMed: 19766645]
- 20.
- Nainan OV, Alter MJ, Kruszon-Moran D, et al. Hepatitis C virus genotypes and viral concentration in participants of a general population survey in the United States. Gastroenterol. 2006;131:478–84. [PubMed: 16890602]
- 21.
- Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461(7262):399–401. [PubMed: 19684573]
- 22.
- Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-α and ribavirin therapy. Nat Genet. 2009;41(10):1100–4. [PubMed: 19749758]
- 23.
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002 Sep 26;347(13):975–82. [PubMed: 12324553]
- 24.
- FDA. Approval of Victrelis (boceprevir) a direct acting antiviral drug (DAA) to treat hepatitis C virus (HCV). 2011. www
.fda.gov/ForConsumers /ByAudience/ForPatientAdvocates /ucm255413.htm. - 25.
- FDA. Approval of Incivek (telaprevir), a direct acting antiviral drug (DAA) to treat hepatitis C (HCV). 2011. www
.fda.gov/ForConsumers /ByAudience/ForPatientAdvocates /ucm256328.htm. - 26.
- Kwo PY, Lawitz EJ, McCone J, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010 Aug 28;376(9742):705–16. [Erratum appears in Lancet. 2010 Oct 9;376(9748):1224 Note: SPRINT-1 investigators [added]; multiple investigator names added] [PubMed: 20692693]
- 27.
- McHutchison JG, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009 Apr 30;360(18):1827–38. [Erratum appears in N Engl J Med. 2009 Oct 8;361(15):1516] [PubMed: 19403902]
- 28.
- Poordad F, McCone J Jr, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011 Mar 31;364(13):1195–206. [PMC free article: PMC3766849] [PubMed: 21449783]
- 29.
- Chou R, Cottrell E, Wasson N. Screening for Hepatitis C Virus Infection in Adults: Comparative Effectiveness Review. Rockville, MD: Agency for Healthcare Research and Quality; 2012. (Prepared by Oregon Evidence-based Practice Center under Contract No. 290-2007-10057-I) Forthcoming. [PubMed: 23304739]
- 30.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–84. [PMC free article: PMC1756728] [PubMed: 9764259]
- 31.
- Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3 Suppl):21–35. [PubMed: 11306229]
- 32.
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann Intern Med. 2011;155(8):529–36. [PubMed: 22007046]
- 33.
- AHRQ. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2011. www
.effectivehealthcare .ahrq.gov/index.cfm /search-for-guides-reviews-and-reports /?pageaction=displayproduct&productid=318. [PubMed: 21959053] - 34.
- Atkins D, Eccles M, Flottorp S, et al. Systems for grading the quality of evidence and the strength of recommendations I: Critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res. 2004;4(1):38. [PMC free article: PMC545647] [PubMed: 15615589]
- 35.
- Fu R, Gartlehner G, Grant M, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the effective health care program. J Clin Epidemiol. 2011 Nov;64(11):1187–97. [PubMed: 21477993]
- 36.
- Kamal SM, Ahmed A, Mahmoud S, et al. Enhanced efficacy of pegylated interferon alpha-2a over pegylated interferon and ribavirin in chronic hepatitis C genotype 4A randomized trial and quality of life analysis. Liver Int. 2011;31(3):401–11. [PubMed: 21281434]
- 37.
- Mach TH, Ciesla A, Warunek W, et al. Efficacy of pegylated interferon alfa-2a or alfa-2b in combination with ribavirin in the treatment of chronic hepatitis caused by hepatitis C virus genotype 1b. Polskie Archiwum Medycyny Wewnetrznej. 2011;121(12):434–40. [PubMed: 22157768]
- 38.
- Yenice N, Mehtap O, Gumrah M, et al. The efficacy of pegylated interferon alpha 2a or 2b plus ribavirin in chronic hepatitis C patients. Turk J Gastroenterol. 2006 Jun;17(2):94–8. [PubMed: 16830289]
- 39.
- Hadziyannis SJ, Sette H Jr, Morgan TR, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004 Mar 2;140(5):346–55. [PubMed: 14996676]
- 40.
- Lagging M, Langeland N, Pedersen C, et al. Randomized comparison of 12 or 24 weeks of peginterferon alpha-2a and ribavirin in chronic hepatitis C virus genotype 2/3 infection. Hepatology. 2008 Jun;47(6):1837–45. [PubMed: 18454508]
- 41.
- Manns M, Zeuzem S, Sood A, et al. Reduced dose and duration of peginterferon alfa-2b and weight-based ribavirin in patients with genotype 2 and 3 chronic hepatitis C. J Hepatol. 2011;55(3):554–63. [PubMed: 21237227]
- 42.
- Shiffman ML, Suter F, Bacon BR, et al. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2007 Jul 12;357(2):124–34. [PubMed: 17625124]
- 43.
- Yu M-L, Dai C-Y, Huang J-F, et al. A randomised study of peginterferon and ribavirin for 16 versus 24 weeks in patients with genotype 2 chronic hepatitis C. Gut. 2007 Apr;56(4):553–9. [PMC free article: PMC1856839] [PubMed: 16956917]
- 44.
- Zeuzem S, Diago M, Gane E, et al. Peginterferon alfa-2a (40 kilodaltons) and ribavirin in patients with chronic hepatitis C and normal aminotransferase levels. Gastroenterol. 2004 Dec;127(6):1724–32. [PubMed: 15578510]
- 45.
- Abergel A, Hezode C, Leroy V, et al. Peginterferon alpha-2b plus ribavirin for treatment of chronic hepatitis C with severe fibrosis: a multicentre randomized controlled trial comparing two doses of peginterferon alpha-2b. J Viral Hepat. 2006 Dec;13(12):811–20. [PubMed: 17109680]
- 46.
- Kawaoka T, Kawakami Y, Tsuji K, et al. Dose comparison study of pegylated interferon-alpha-2b plus ribavirin in naive Japanese patients with hepatitis C virus genotype 2: a randomized clinical trial. J Gastroenterol Hepatol. 2009 Mar;24(3):366–71. [PubMed: 19032459]
- 47.
- Krawitt EL, Gordon SR, Grace ND, et al. A study of low dose peginterferon alpha-2b with ribavirin for the initial treatment of chronic hepatitis C. Am J Gastroenterol. 2006 Jun;101(6):1268–73. [PubMed: 16771948]
- 48.
- Meyer-Wyss B, Rich P, Egger H, et al. Comparison of two PEG-interferon alpha-2b doses (1.0 or 1.5 microg/kg) combined with ribavirin in interferon-naive patients with chronic hepatitis C and up to moderate fibrosis. J Viral Hepat. 2006 Jul;13(7):457–65. [PubMed: 16792539]
- 49.
- Sood A, Midha V, Hissar S, et al. Comparison of low-dose pegylated interferon versus standard high-dose pegylated interferon in combination with ribavirin in patients with chronic hepatitis C with genotype 3: an Indian experience. J Gastroenterol Hepatol. 2008 Feb;23(2):203–7. [PubMed: 17645472]
- 50.
- Jacobson IM, Brown RS Jr, Freilich B, et al. Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: a randomized trial. Hepatology. 2007 Oct;46(4):971–81. [PubMed: 17894303]
- 51.
- Magni CNF, Argenteri B, Giorgi R, Mainini A, Pastecchia C, Ricci E, Schiavini M, Terzi R, Vivirito MC, Resta M. Abstract #883: Antiviral activity and tolerability between pegylated interferon alpha 2a and alpha 2b in naive patients with chronic hepatitis C: Results of a prospective monocentric randomized trial. Hepatology. 2009;50(S4):720A.
- 52.
- Miyase S, Haraoka K, Ouchida Y, et al. Randomized trial of peginterferon α-2a plus ribavirin versus peginterferon α-2b plus ribavirin for chronic hepatitis C in Japanese patients. J Gastroenterol. 2012:1–8. [PubMed: 22382633]
- 53.
- Hezode C, Forestier N, Dusheiko G, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009 Apr 30;360(18):1839–50. [PubMed: 19403903]
- 54.
- Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–16. [PubMed: 21696307]
- 55.
- Kumada H, Toyota J, Okanoue T, et al. Telaprevir with peginterferon and ribavirin for treatment-naive patients chronically infected with HCV of genotype 1 in Japan. J Hepatol. 2012;56(1):78–84. [PubMed: 21827730]
- 56.
- Marcellin P, Forns X, Goeser T, et al. Telaprevir is effective given every 8 or 12 hours with ribavirin and peginterferon alfa-2a or -2b to patients with chronic hepatitis C. Gastroenterol. 2011 Feb;140(2):459–68.e1. quiz e14. [PubMed: 21034744]
- 57.
- Sherman KE, Flamm SL, Afdhal NH, et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365(11):1014–24. [PMC free article: PMC3809077] [PubMed: 21916639]
- 58.
- Arase Y, Ikeda K, Suzuki F, et al. Long-term outcome after interferon therapy in elderly patients with chronic hepatitis C. Intervirology. 2007;50(1):16–23. [PubMed: 17164553]
- 59.
- Bruno S, Stroffolini T, Colombo M, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007 Mar;45(3):579–87. [PubMed: 17326216]
- 60.
- Cardoso A-C, Moucari R, Figueiredo-Mendes C, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol. 2010 May;52(5):652–7. [PubMed: 20346533]
- 61.
- Coverdale SA, Khan MH, Byth K, et al. Effects of interferon treatment response on liver complications of chronic hepatitis C: 9-year follow-up study. Am J Gastroenterol. 2004 Apr;99(4):636–44. [PubMed: 15089895]
- 62.
- El Braks RE, Ganne-Carrie N, Fontaine H, et al. Effect of sustained virological response on long-term clinical outcome in 113 patients with compensated hepatitis C-related cirrhosis treated by interferon alpha and ribavirin. World J Gastroenterol. 2007;13(42):5648–53. [PMC free article: PMC4172746] [PubMed: 17948941]
- 63.
- Fernández-Rodríguez CM, Alonso S, Martinez SM, et al. Peginterferon plus ribavirin and sustained virological response in HCV-related cirrhosis: Outcomes and factors predicting response. Am J Gastroenterol. 2010 [PubMed: 20700116]
- 64.
- Hasegawa E, Kobayashi M, Kawamura Y, et al. Efficacy and anticarcinogenic activity of interferon for hepatitis C virus-related compensated cirrhosis in patients with genotype 1b low viral load or genotype 2. Hepatol Res. 2007;37(10):793–800. [PubMed: 17593231]
- 65.
- Hung CH, Lee CM, Lu SN, et al. Long-term effect of interferon alpha-2b plus ribavirin therapy on incidence of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. J Viral Hepat. 2006;13(6):409–14. [PubMed: 16842444]
- 66.
- Imazeki F, Yokosuka O, Fukai K, et al. Favorable prognosis of chronic hepatitis C after interferon therapy by long-term cohort study. Hepatology. 2003 Aug;38(2):493–502. [PubMed: 12883494]
- 67.
- Innes HA, Hutchinson SJ, Allen S, et al. Excess liver-related morbidity of chronic hepatitis C patients, who achieve a sustained viral response, and are discharged from care. Hepatology. 2011;54(5):1547–58. [PubMed: 22045672]
- 68.
- Izumi N, Yasuhiro A, Kurosaki M, et al. Development of hepatocellular carcinoma after interferon therapy in chronic hepatitis C. Is it possible to reduce the incidence by ribanirin and IFN combination therapy? Intervirology. 2005;48(1):59–63. [PubMed: 15785091]
- 69.
- Kasahara A, Tanaka H, Okanoue T, et al. Interferon treatment improves survival in chronic hepatitis C patients showing biochemical as well as virological responses by preventing liver-related death. J Viral Hepat. 2004 Mar;11(2):148–56. [PubMed: 14996350]
- 70.
- Maruoka D, Imazeki F, Arai M, et al. Long-term cohort study of chronic hepatitis C according to interferon efficacy. J Gastroenterol Hepatol. 2012;27(2):291–9. [PubMed: 21793911]
- 71.
- Shiratori Y, Ito Y, Yokosuka O, et al. Antiviral therapy for cirrhotic hepatitis C: association with reduced hepatocellular carcinoma development and improved survival. Ann Intern Med. 2005 Jan 18;142(2):105–14. [PubMed: 15657158]
- 72.
- Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147(10):677–84. [PubMed: 18025443]
- 73.
- Yoshida H, Arakawa Y, Sata M, et al. Interferon therapy prolonged life expectancy among chronic hepatitis C patients. Gastroenterol. 2002 Aug;123(2):483–91. [PubMed: 12145802]
- 74.
- Yu M-L, Lin S-M, Chuang W-L, et al. A sustained virological response to interferon or interferon/ribavirin reduces hepatocellular carcinoma and improves survival in chronic hepatitis C: a nationwide, multicentre study in Taiwan. Antiver Ther. 2006;11(8):985–94. [PubMed: 17302368]
- 75.
- Ferenci P, Brunner H, Laferl H, et al. A randomized, prospective trial of ribavirin 400 mg/day versus 800 mg/day in combination with peginterferon alfa-2a in hepatitis C virus genotypes 2 and 3. Hepatology. 2008 Jun;47(6):1816–23. [PubMed: 18454510]
- 76.
- Helbling B, Jochum W, Stamenic I, et al. HCV-related advanced fibrosis/cirrhosis: randomized controlled trial of pegylated interferon alpha-2a and ribavirin. J Viral Hepat. 2006 Nov;13(11):762–9. [PubMed: 17052276]
- 77.
- Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–65. [PubMed: 11583749]
- 78.
- Shibuya K, Yano E. Regression analysis of trends in mortality from hepatocellular carcinoma in Japan, 1972–2001. Int J Epidemiol. 2005;34(2):397–402. [PubMed: 15561746]
- 79.
- Singal AK, Jampana SC, Anand BS. Peginterferon alfa-2a is superior to peginterferon alfa-2b in the treatment of naïve patients with hepatitis C virus infection: meta-analysis of randomized controlled trials. Dig Dis Sci. 2011 Aug;56(8):2221–6. [PubMed: 21643737]
- 80.
- Singal AK, Anand BS. Tailoring treatment duration to 12 to 16 weeks in hepatitis C genotype 2 or 3 with rapid virologic response: systematic review and meta-analysis of randomized controlled trials. J Clin Gastroenterol. 2010;44(8):583–7. [PubMed: 20375729]
- 81.
- Mangia A, Santoro R, Minerva N, et al. Peginterferon alfa-2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2005 Jun 23;352(25):2609–17. [PubMed: 15972867]
- 82.
- Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis. 2011;52(7):889–900. [PubMed: 21427396]
- 83.
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–27. [PubMed: 21992124]
- 84.
- Ghany MG, Kim H-Y, Stoddard A, et al. Predicting clinical outcomes using baseline and follow-up laboratory data from the hepatitis C long-term treatment against cirrhosis trial. Hepatology. 2011 Nov;54(5):1527–37. [PMC free article: PMC3718548] [PubMed: 22045670]
- 85.
- PEGASYS [package insert]. Hoffmann-La Roche Inc.; Nutley, NJ: 2002.
- 86.
- PEG-Intron [package insert]. Schering Corporation; Kenilworth, NJ: 2005.
- 87.
- Morrison A, Moulton K, Clark M, et al. English-Language Restriction When Conducting Systematic Review-based Metaanalyses: Systematic Review of Published Studies. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2009. http://journals
.cambridge .org/action/displayAbstract?fromPage =online&aid=8544780. - 88.
- Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. British Medical Journal. 2011 Jul;343(1):d4002. 2011. [PubMed: 21784880]
- 89.
- Lok A. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366(3):273–5. [PubMed: 22256805]
- Executive Summary - Treatment for Hepatitis C Virus Infection in AdultsExecutive Summary - Treatment for Hepatitis C Virus Infection in Adults
Your browsing activity is empty.
Activity recording is turned off.
See more...
