NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Chou R, Hartung D, Rahman B, et al. Treatment for Hepatitis C Virus Infection in Adults [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012 Nov. (Comparative Effectiveness Reviews, No. 76.)
Hepatitis C virus (HCV) is the most common chronic bloodborne pathogen in the United States. HCV is primarily acquired by large or repeated percutaneous exposures to blood, with injection drug use the strongest risk factor. Based on a national survey of households, approximately 1.6 percent of U.S. adults over 20 years of age have antibodies to HCV, indicating prior acute HCV infection.1 About 78 percent of patients with acute HCV infection develop chronic HCV infection, defined by the presence of persistent viremia.
Chronic HCV infection has a variable course, but it is a leading cause of complications from chronic liver disease, including cirrhosis, liver failure, and hepatocellular carcinoma (HCC). Chronic HCV infection was associated with an estimated 15,000 deaths in the United States in 2007,2 and it is the most common indication for liver transplantation among American adults, accounting for more than 30 percent of cases.3 The prevalence of chronic HCV infection is thought to have peaked in 2001 at 3.6 million people, and the yearly incidence has declined from more than 200,000 cases per year in the 1980s to around 16,000 cases in 2009.4, 5 However, complications related to chronic HCV infection, which frequently occur only after decades of infection, are expected to rise for another 10 to 13 years.4
The goals of antiviral treatment for chronic HCV infection are to prevent the long-term health complications associated with HCV infection, such as cirrhosis, hepatic decompensation, and liver cancer, but it is a challenge to design and carry out clinical trials long and large enough to provide direct evidence related to these outcomes. The sustained virologic response (SVR) rate, typically defined as a decline in HCV-RNA (Hepatitis C virus ribonucleic acid) to undetectable levels 24 weeks following completion of antiviral treatment, is the standard marker for successful treatment in clinical trials because it is strongly associated with long-term absence of viremia.6, 7 Recent studies have evaluated the association between achieving an SVR and reductions in mortality, liver failure, and cancer.8, 9
The treatment of HCV infection has evolved dramatically over the past several decades. Recombinant type I interferons were introduced as monotherapy in the mid-1980s, but were only modestly successful at achieving SVR (overall <20 percent).10–13 Subsequent trials found dual therapy with interferon and the synthetic nucleoside analogue ribavirin more effective than monotherapy with interferon, although the SVR rates remained under 50 percent.10–13
In the early 2000s, the combination of “pegylated” interferon plus ribavirin became the standard antiviral treatment for HCV infection.14–16 The first pegylated interferon was approved by the FDA in 2001. Pegylation refers to the cross-linking of polyethylene glycol molecules to the interferon molecule, which delays renal clearance and thereby permits less frequent dosing (once weekly vs. three times a week with nonpegylated interferon).17 Currently, two pegylated interferons are available: pegylated interferon alfa-2a and pegylated interferon alfa-2b. Both are Type I alfa interferons, but differ in the size and structure of the interferon and polyethylene glycol molecules, as well as in their pharmacokinetic properties (Table 1).17 One pegylated interferon consists of 31-kilodalton (kDa) interferon alfa-2b conjugated to 12-kDa polyethylene glycol (brand name PEG-intron®). The other consists of recombinant 20-kDa interferon alfa-2a linked to 40-kDa polyethylene glycol (trade name Pegasys®). The dosing schedule is fixed for pegylated interferon alfa-2a and is based on weight for pegylated interferon alfa-2b. Each pegylated interferon is approved for dual therapy with ribavirin. Although each pegylated interferon is approved for combination therapy with a specific brand of ribavirin manufactured by the respective manufacturer (Copegus® for pegylated interferon alfa-2a and Rebetol® for alfa-2b), the ribavirin is pharmacologically identical. The FDA-recommended doses of ribavirin are 800 to 1200 mg/day for pegylated interferon alfa-2a, depending on weight and genotype, and 800 to 1400 mg/day for pegylated interferon alfa-2b, depending on weight.
Table 1
Pharmacokinetics, indications, and dosing of included drugs.
Dual therapy with pegylated interferon (alfa-2a or alfa-2b) plus ribavirin is associated with higher SVR rates (about 55–60 percent overall) than either nonpegylated interferon plus ribavirin or pegylated interferon (alfa-2a or alfa-2b) monotherapy. Although previous reviews found insufficient evidence to determine whether dual therapy with pegylated interferon alfa-2a or pegylated interferon alfa-2b is more effective,18, 19 more head-to-head trials directly comparing these two regimens are now available.20–23
A number of factors affect response to antiviral treatment. The two major pretreatment predictors of SVR are the viral genotype and the pretreatment viral load.15 In the United States, genotype 1 infection is found in around three-quarters of HCV-infected patients.24 HCV genotype 1 infection is associated with a substantially lower response to antiviral treatment than infection with genotypes 2 and 3, which are present in about 20 percent of HCV-infected patients. A pretreatment viral load of <600,000 international units per milliliter (IU/mL) is associated with higher likelihood of achieving an SVR.15 Other factors less consistently or less strongly associated with increased likelihood of SVR include female sex, age less than 40 years, non-Black race, lower body weight (≤75 kg), absence of insulin resistance, elevated alanine aminotransferase (ALT) levels, and absence of bridging fibrosis or cirrhosis on liver biopsy.15 Effects of race on the likelihood of SVR may be due in part to polymorphisms in the interleukin-28B (IL28B) gene.25, 26
An issue complicating antiviral treatment is the high rate of adverse effects observed with interferon-based therapy, including flulike symptoms, fatigue, and neuropsychiatric and hematologic adverse effects.27 Such adverse effects can be difficult to tolerate and can lead to premature discontinuation of therapy.
In 2011, the U.S. Food and Drug Administration (FDA) approved the first direct acting antiviral agents, boceprevir (trade name Victrelis®) and telaprevir (trade name Incivek®), for treatment of chronic HCV genotype 1 infection (Table 1).28, 29 Both drugs are classified as nonstructural (NS) 3/4A protease inhibitors, with a potential advantage of shorter duration of therapy (24 to 28 weeks) when used in combination with pegylated interferon (alfa-2a or alfa-2b) compared with standard dual therapy with pegylated interferon (alfa-2a plus -2b) plus ribavirin for genotype 1 infection (48 weeks) (Table 1).30–32
Understanding the comparative benefits and harms of the various antiviral regimens is critical for making informed treatment decisions in patients with chronic HCV infection, particularly given the availability of new treatment options. This review will assess the comparative effectiveness of antiviral treatments in adults with chronic HCV infection who have not received previous antiviral drug treatment. In addition to assessing the comparative effectiveness of different drug regimens, the review will evaluate effects of different medication doses, durations of therapy, and dosing strategies (such as weight-based or response-guided vs. fixed treatment). To help with individualized clinical decisionmaking regarding antiviral therapy for chronic HCV infection, it will also evaluate how comparative effectiveness varies depending on HCV genotype, viral load, and other demographic and clinical characteristics. Because estimating potential benefits and harms of HCV screening requires an understanding of the effects of treatment in people with HCV infection, this review will be used, together with a separate review on HCV screening,35 by the U.S. Preventive Services Task Force to update its HCV screening recommendations.
Scope and Key Questions
The analytic framework and Key Questions used to guide this report are shown below (Figure 1). The analytic framework shows the target populations, interventions, and intermediate and health outcome measures we examined.
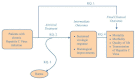
Figure 1
Analytic framework for treatment of hepatitis C infection in adults. KQ = Key Question
The following Key Questions are the focus of our report:
Key Question 1
- What is the comparative effectiveness of antiviral treatment in improving health outcomes in patients with HCV infection?
- How does the comparative effectiveness of antiviral treatment for health outcomes vary according to patient subgroup characteristics, including but not limited to HCV genotype, age, race, sex, stage of disease, or genetic markers?
Key Question 2
- What is the comparative effectiveness of antiviral treatments on intermediate outcomes, such as the rate of SVR or histologic changes in the liver?
- How does the comparative effectiveness of antiviral treatment for intermediate outcomes vary according to patient subgroup characteristics, including but not limited to HCV genotype, age, race, sex, stage of disease, or genetic markers?
Key Question 3
- What are the comparative harms associated with antiviral treatments?
- Do these harms differ according to patient subgroup characteristics, including HCV genotype, age, race, sex, stage of disease, or genetic markers?
Key Question 4
Have improvements in intermediate outcomes (SVR, histologic changes) been shown to reduce the risk or rates of adverse health outcomes from HCV infection?
Key Question 1 focuses on direct evidence on the comparative effectiveness of antiviral treatments for chronic HCV infection on health outcomes (such as death, cirrhosis, hepatic decompensation, HCC, need for transplantation, or quality of life). Because of the long duration (typically decades) necessary develop major hepatic complications related to chronic HCV infection, it is difficult to assess for such outcomes in clinical trials. In addition, dual therapy with pegylated interferon (alfa-2a or alfa-2b) plus ribavirin has only been available since 2001, and protease inhibitors only became approved by the FDA in 2011, which might not be enough time to adequately evaluate some long-term clinical outcomes. Therefore, Key Question 2 focuses on evidence on the comparative effectiveness of antiviral treatments for chronic HCV infection on intermediate outcomes (SVR and histological improvements). Key Question 4 assesses the link between intermediate and clinical outcomes, in order to facilitate interpretation of results obtained for Key Question 2. Key Question 3 focuses on the comparative harms of different antiviral treatments.
- Introduction - Treatment for Hepatitis C Virus Infection in AdultsIntroduction - Treatment for Hepatitis C Virus Infection in Adults
Your browsing activity is empty.
Activity recording is turned off.
See more...
