NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Comparative Effectiveness Review Summary Guides for Clinicians [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2007-.
This publication is provided for historical reference only and the information may be out of date.
Research Focus for Clinicians
Among the many medications for antihypertensive therapy are those aimed at inhibiting or blocking the renin-angiotensin system (RAS). A systematic review of 110 clinical studies published between 1988 and 2010 sought to determine the comparative effectiveness, benefits, and adverse effects of angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), and a direct renin inhibitor (DRI) for adults with hypertension. The report does not review studies comparing: individual drugs within each class, evidence about using these drugs for congestive heart failure or diabetic kidney disease, switching from one drug class to another, or combination therapy. This summary is provided to assist in decisionmaking along with a patient’s values and preferences. Reviews of evidence should not be construed to represent clinical recommendations or guidelines. The full report, listing all studies, is available at www.effectivehealthcare.ahrq.gov/acearbhbp.cfm.
Background
Hypertension-related morbidity can be reduced with effective lifestyle interventions, including diet, exercise, and control of body weight; however, many people require antihypertensive medications to lower their blood pressure.
ACEIs, ARBs, and the DRI aliskiren are approved for treating hypertension in adults. While all three drug classes target the RAS (see figure 1), it is not clear that these medications are clinically equivalent.
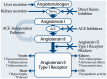
Figure 1
Different mechanisms of pharmacological blockade of the renin-angiotensin system.
The evidence on the comparative long-term benefits and adverse effects of ACEIs, ARBs, and the DRI aliskiren is summarized here, focusing on their use for treating hypertension in adults. This summary is an update of a 2007 report that evaluated the scientific literature on ACEIs and ARBs for adults with hypertension. This update adds evaluation of the DRI aliskiren.
Conclusions
There is a high strength of evidence that ACEIs and ARBs control blood pressure to a similar extent. Data are limited for comparisons involving the DRI aliskiren. ACEIs and ARBs have similar effects on mortality, major cardiovascular events, quality of life, lipid levels, markers of carbohydrate metabolism/ diabetes control, and other adverse events excluding cough. Cough is significantly higher with ACEIs when compared with an ARB. For many outcomes, comparisons of ACEIs or ARBs with the DRI aliskiren were not evaluated, precluding meaningful conclusions about aliskiren.
Clinical Bottom Line
| Evidence of Benefits |
|---|
Similar long-term blood pressure-lowering effects were seen with ACEIs and ARBs.
 The DRI aliskiren may be slightly more effective at reducing blood pressure than an ACEI (ramipril); however, no differences were detected between aliskiren and an ARB (losartan).  |
No significant differences were found between ACEIs and ARBs for these outcomes:
|
ACEIs and ARBs are similar in their lack of effect on serum lipid levels, blood glucose levels, and HbA1c.
 |
Evidence was insufficient for all other outcomes beyond blood pressure reduction on the comparative effectiveness of the DRI aliskiren.
 |
| Evidence of Adverse Effects |
Cough is more prevalent in patients on ACEIs than those on ARBs (About 9% of patients treated with an ACEI and about 2% of patients treated with an ARB report a cough).
 |
ACEIs were associated with lower rates of persistence and higher rates of withdrawals due to adverse events when compared with ARBs.
 |
Angioedema was uncommon and most frequently associated with ACEIs.
 |
HbA1c = hemoglobin A1c; LVEF = left ventricular ejection fraction; LVMI = left ventricular mass index
Strength of Evidence Scale
High:
 There are consistent results from good-quality studies. Further research is very unlikely to change the conclusions.
There are consistent results from good-quality studies. Further research is very unlikely to change the conclusions.Moderate:
 Findings are supported, but further research could change the conclusions.
Findings are supported, but further research could change the conclusions.Low:
 There are very few studies, or existing studies are flawed.
There are very few studies, or existing studies are flawed.Insufficient:
 Research is either unavailable or does not permit estimation of a treatment effect.
Research is either unavailable or does not permit estimation of a treatment effect.
Additional Information on Adverse Effects
- In one study, aliskiren was associated with angioedema in one patient, but overall the evidence was insufficient.
- Excluding cough, there were no significant between-class differences in any other adverse events.
- Lower persistence with ACEIs versus ARBs may be explained largely by the differential rates of cough.
- When used during pregnancy, ACEIs, ARBs, and the DRI aliskiren can injure or be fatal to the developing fetus.
Gaps in Knowledge
- Long-term comparisons of the DRI aliskiren with ACEIs and ARBs.
- The impact of cough on quality of life, care patterns (e.g., use of therapeutic agents for cough symptoms or conditions associated with cough), and health outcomes, particularly for individuals who continue to use ACEIs.
- Subgroups of special importance such as individuals with hypertension and diabetes mellitus, congestive heart failure, chronic kidney disease, and dyslipidemia.
- Broader representation of groups such as the elderly and ethnic and racial minorities.
- Clinical trials with long-term followup that report on the incidence of new cancer diagnoses and cancer deaths among patients on ACEIs, ARBs, or a DRI.
Patient Cost Information
- Medication costs may contribute to decreased adherence among patients. When prescribing, discuss his or her health insurance coverage and budget limitations. Considering medication costs when selecting antihypertensive agents may be warranted.
- Average wholesale prices for antihypertensive agents range from $30 to $160 per month, depending on dosage.
- On average, ACEIs are less expensive for patients than ARBs and the DRI aliskiren.
- The most inexpensive ACEIs for patients are the generic forms of benazepril, enalapril, lisinopril, and quinapril.
- The majority of ARBs are not available in generic form. The average cost of most brand-name ARBs is between $80 and $195 per month, depending on dosage. The DRI aliskiren is also not available in a generic form. The average wholesale cost of aliskiren is $100 or $120 per month, depending on dosage.
What To Discuss With Your Patients
- The importance of taking blood pressure medication as prescribed.
- The tradeoffs between the benefits and adverse effects when taking an ACEI, an ARB, or a DRI.
- How to identify and when to report serious side effects.
- Barriers that may affect adherence to their specific treatment regimen.
- All other medications they may be taking and possible interactions with their blood pressure medications.
Resource for Patients
Choosing Medicines for High Blood Pressure: A Review of the Research on ACEIs, ARBs, and DRIs is a companion to this clinician research summary. It can help people talk with their health care professionals about the effectiveness, side effects, and cost of ACEIs, ARBs, and DRI.
Ordering Information
For electronic copies of the consumer research review, this clinician research summary, and the full systematic review, visit www.effectivehealthcare.ahrq.gov/acearbhbp.cfm. To order free print copies, call the AHRQ Publications Clearinghouse at 800-358-9295.
Source
The information in this summary is based on Angiotensin-Converting Enzyme Inhibitors (ACEIs), Angiotensin II Receptor Antagonists (ARBs), and Direct Renin Inhibitors for Treating Essential Hypertension: An Update, Comparative Effectiveness Review No. 34, prepared by the Duke Evidence-based Practice Center under Contract No. 290-02-0025 for the Agency for Healthcare Research and Quality (AHRQ), July 2011. Available at: www.effectivehealthcare.ahrq.gov/acearbhbp.cfm. This summary was prepared by the John M. Eisenberg Center for Clinical Decisions and Communications Science at Baylor College of Medicine, Houston, TX.
- Review Comparing Medications for Adults With Type 2 Diabetes.[Comparative Effectiveness Revi...]Review Comparing Medications for Adults With Type 2 Diabetes.John M. Eisenberg Center for Clinical Decisions and Communications Science. Comparative Effectiveness Review Summary Guides for Clinicians. 2007
- Review Angiotensin-converting enzyme inhibitors (ACEIs), not angiotensin receptor blockers (ARBs), are preferred and effective mode of therapy in high cardiovascular risk patients.[J Indian Med Assoc. 2009]Review Angiotensin-converting enzyme inhibitors (ACEIs), not angiotensin receptor blockers (ARBs), are preferred and effective mode of therapy in high cardiovascular risk patients.Vijan SG. J Indian Med Assoc. 2009 Mar; 107(3):178-82.
- Review Perioperative angiotensin-converting enzyme inhibitors or angiotensin II type 1 receptor blockers for preventing mortality and morbidity in adults.[Cochrane Database Syst Rev. 2016]Review Perioperative angiotensin-converting enzyme inhibitors or angiotensin II type 1 receptor blockers for preventing mortality and morbidity in adults.Zou Z, Yuan HB, Yang B, Xu F, Chen XY, Liu GJ, Shi XY. Cochrane Database Syst Rev. 2016 Jan 27; 2016(1):CD009210. Epub 2016 Jan 27.
- Review Analgesics for Osteoarthritis.[Comparative Effectiveness Revi...]Review Analgesics for Osteoarthritis.John M Eisenberg Center for Clinical Decisions and Communications Science. Comparative Effectiveness Review Summary Guides for Clinicians. 2007
- Review Nonpharmacologic Interventions for Treatment-Resistant Depression in Adults.[Comparative Effectiveness Revi...]Review Nonpharmacologic Interventions for Treatment-Resistant Depression in Adults.John M. Eisenberg Center for Clinical Decisions and Communications Science. Comparative Effectiveness Review Summary Guides for Clinicians. 2007
- ACEIs, ARBs, or DRI for Adults With Hypertension - Comparative Effectiveness Rev...ACEIs, ARBs, or DRI for Adults With Hypertension - Comparative Effectiveness Review Summary Guides for Clinicians
Your browsing activity is empty.
Activity recording is turned off.
See more...
