NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Comparative Effectiveness Review Summary Guides for Clinicians [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2007-.
This publication is provided for historical reference only and the information may be out of date.
Clinical Issue
Off-label use of recombinant activated factor VII (rFVIIa) in the hospital setting has increased despite limited comparative data on its effectiveness and safety. How is it being used, and what is the evidence of benefits and harms for treated patients?
The U.S. Food and Drug Administration has approved rFVIIa for the treatment and prevention of bleeding in patients with hemophilia A or B with inhibitors, acquired hemophilia, or congenital factor VII deficiency. However, in recent years the off-label use of rFVIIa has increased in the hospital setting to prevent or manage uncontrolled bleeding. The most common in-hospital, off-label uses of rFVIIa are for spontaneous intracranial hemorrhage, bleeding secondary to trauma, and cardiac surgery (see Fig. 1 on the reverse).
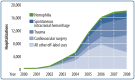
Figure 1
Growth of in-hospital, off-label vs. on-label uses of rFVIIa in Premier database, 2000–2008*. * The unit of analysis was any hospital “case” of rFVIIa use—defined as any application during a patient hospitalization.
Clinical Bottom Line
| In-Hospital, Off-Label Uses for rFVIIa1 | Evidence on Benefits vs. Usual Care | Evidence on Harms vs. Usual Care |
|---|---|---|
| Spontaneous intracranial hemorrhage* | No effect on
mortality or functional status.  Attenuation of hematoma expansion.  | Increased
risk for arterial thromboembolic events, particularly at doses
>40 μg/kg of patient body weight.†
 |
| Adult cardiac surgery | No effect on
mortality, RBC transfusion requirements, or ICU length of stay.
 | Increased
risk for thromboembolic events when compared to usual care.
 |
| Body trauma* | No consistent
effect on mortality.  Possible reduction in acute respiratory distress syndrome.  | No effect on
thromboembolic events.  |
| Brain trauma | No effect on
mortality, Glasgow coma scale, or hematoma volume change.
 | No effect on
thromboembolic events.  |
| Liver transplantation* | No effect on
mortality, OR time, or ICU length of stay.  Possible reduction in 24-hr RBC transfusion requirements.  | No effect on
thromboembolic events.  |
- 1
In January 2010, an FDA-required warning statement regarding the serious thrombotic events associated with the use of rFVIIa (NovoSeven® RT) outside labeled indications was added to the product insert. This warning was similar to that issued in 2005 for the original formulation of rFVIIa (NovoSeven®).
- *
Current evidence does not provide a connection between improvements in indirect outcomes (e.g., reversal of bleeding) with those of direct outcomes (e.g., mortality). Explanations include: 1) bleeding control by itself is not enough to influence direct outcomes, or 2) the risk of overall harms outweighs the potential benefits from indirect outcomes.
- †
Even though the effect of treating spontaneous intracranial hemorrhage with rFVIIa at doses ≤40 μg/kg of patient body weight was not statistically different from zero, there may have been insufficient statistical power to detect a difference.
Confidence Scale
High:
 There are consistent results from good-quality studies. Further research is very
unlikely to change the conclusions.
There are consistent results from good-quality studies. Further research is very
unlikely to change the conclusions.
Moderate:
 Findings are supported, but further research could change the conclusions.
Findings are supported, but further research could change the conclusions.
Low:
 There are very few studies, or existing studies are flawed.
There are very few studies, or existing studies are flawed.
Conclusions
For the uses examined, current evidence does not show that off-label use of rFVIIa reduces mortality or improves other direct outcomes. Thromboembolic events are increased by use of rFVIIa to treat spontaneous intracranial hemorrhage and in adult cardiac surgery.
Additional Details Regarding Increased Risk of Thromboembolic Events* With rFVIIa Use for Spontaneous Intracranial Hemorrhage and Adult Cardiac Surgery
| In-Hospital, Off-Label Use of rFVIIa | TE Events/Total Patients (%) | Risk Difference Summary Effect Size (95% CI) | Estimated Effect on TE Events | |
|---|---|---|---|---|
| rFVIIa | Usual Care | |||
| Spontaneous intracranial hemorrhage | ||||
| Low dose (≤40 μg/kg) | 24/415 (5.8) | 13/378 (3.4) | 0.025 (−0.004 to 0.053) | No
effect†
 |
| Medium dose (41–119 μg/kg) | 29/399 (7.3) | 13/378 (3.4) | 0.035 (0.008 to 0.062) | Increase
with rFVIIa  |
| High dose (≥120 μg/kg) | 8/115 (7.0) | 0/107 (0) | 0.063 (0.011 to 0.114) | Increase
with rFVIIa  |
| Adult cardiac surgery ‡ | 19/203 (9.4) | 8/170 (4.7) | 0.053 (0.01 to 0.096) | Increase
with rFVIIa  |
- *
Data for spontaneous intracranial hemorrhage includes only arterial thromboembolic events. Data for adult cardiac surgery includes all reported thromboembolic events as venous and arterial events were not reported separately.
- †
Even though the effect of treating spontaneous intracranial hemorrhage with rFVIIa at doses ≤40 μg/kg of patient body weight was not statistically different from zero, there may have been insufficient statistical power to detect a difference.
- ‡
The dose used in cardiac surgery was on the lower end of those studied (17–90 μg/kg) and was typically only given in the form of 1–2 infusions, rather than multiple infusions seen in some of the other indications.
CI = confidence interval; TE = thromboembolic.
Additional Off-Label Uses of rFVIIa Requiring Future Research
Surgery
- Management of abdominal aortic aneurysm (with and without surgical intervention).
- Pediatric cardiac surgery.
- Surgical procedures beyond cardiac and vascular surgery.
- Vascular surgeries (not related to abdominal aortic aneurysm).
Medical Conditions
- Cancer-related conditions.
- Gastrointestinal bleeding not related to liver disease.
- Hematopoietic stem cell transplantation.
- Liver disease (other than transplantation).
- Neonatal conditions (beyond cardiac surgery).
- Obstetrical conditions.
- Primary clotting disorders (other than hemophilia).
- Pulmonary conditions (e.g., pulmonary hemorrhage and pulmonary transplantation).
- Secondary clotting disorders (e.g., complications of warfarin anticoagulation).
Source
The information in this guide is based on Comparative Effectiveness of In-Hospital Use of Recombinant Factor VIIa for Off-Label Indications vs. Usual Care, Comparative Effectiveness Review No. 21, prepared by the Stanford–UCSF Evidence-based Practice Center under Contract No. 290-02-0017 for the Agency for Healthcare Research and Quality, May 2010. Available at: www.effectivehealthcare.ahrq.gov. This summary was prepared by the John M. Eisenberg Center for Clinical Decisions and Communications Science at Baylor College of Medicine, Houston, Texas.
A note about this Clinician Guide
A systematic review of 74 clinical studies was conducted by independent researchers, funded by AHRQ, to synthesize the evidence on what is known and not known on this clinical issue.
This topic was nominated through a public process. The research questions and the results of the report were subject to expert input, peer review, and public comment.
The results of this review are summarized here for use in your decisionmaking and in discussions with patients. The full report, with references for included and excluded studies, is available at www
.effectivehealthcare.ahrq.gov.
- Off-label use of recombinant factor VIIa in U.S. hospitals: analysis of hospital records.[Ann Intern Med. 2011]Off-label use of recombinant factor VIIa in U.S. hospitals: analysis of hospital records.Logan AC, Yank V, Stafford RS. Ann Intern Med. 2011 Apr 19; 154(8):516-22.
- Review Comparative Effectiveness of In-Hospital Use of Recombinant Factor VIIa for Off-Label Indications vs. Usual Care[ 2010]Review Comparative Effectiveness of In-Hospital Use of Recombinant Factor VIIa for Off-Label Indications vs. Usual CareYank V, Tuohy CV, Logan AC, Bravata DM, Staudenmayer K, Eisenhut R, Sundaram V, McMahon D, Stave CD, Zehnder JL, et al. 2010 May
- Review Intraoperative use of recombinant activated factor VII (rFVIIa).[Minerva Anestesiol. 2006]Review Intraoperative use of recombinant activated factor VII (rFVIIa).De Gasperi A. Minerva Anestesiol. 2006 Jun; 72(6):489-94.
- Review The Judicious Use of Recombinant Factor VIIa.[Semin Thromb Hemost. 2016]Review The Judicious Use of Recombinant Factor VIIa.Goodnough LT, Levy JH. Semin Thromb Hemost. 2016 Mar; 42(2):125-32. Epub 2016 Feb 2.
- Review Recombinant factor VIIa: an assessment of evidence regarding its efficacy and safety in the off-label setting.[Hematology Am Soc Hematol Educ...]Review Recombinant factor VIIa: an assessment of evidence regarding its efficacy and safety in the off-label setting.Logan AC, Goodnough LT. Hematology Am Soc Hematol Educ Program. 2010; 2010:153-9.
- Utilization and Clinical Data on In-Hospital, Off-Label Uses of Recombinant Fact...Utilization and Clinical Data on In-Hospital, Off-Label Uses of Recombinant Factor VIIa - Comparative Effectiveness Review Summary Guides for Clinicians
Your browsing activity is empty.
Activity recording is turned off.
See more...
