NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Dean L, McEntyre J, editors. Coffee Break: Tutorials for NCBI Tools [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 1999-.
Tumors are formed by an abnormal proliferation of undifferentiated cells. At the molecular level, this represents a failure to adequately control cell growth and division.
In normal cells, there are many genes that code for regulatory proteins, which are responsible for maintaining the delicate balance required for cell division to proceed at the right time and in the right place. Among these, proto-oncogenes stimulate the cell division cycle, whereas tumor suppressor genes act as brakes. When these types of genes fail to do their jobs, perhaps as a result of a mutation, the control mechanisms break down and cancerous growth can ensue.
Because many proto-oncogenes are kinases (enzymes that have a stimulatory effect in cell signaling pathways), the existence of a tumor suppressor gene that acts as a phosphatase (an enzyme that counteracts the action of kinases) was predicted. However, it was almost 10 years after the discovery of the retinoblastoma gene, the first tumor suppressor to be described, that a gene product answering to this phosphatase description was found.
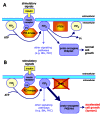
The PTEN gene, located on chromosome 10q23, is missing or mutated in a variety of human cancers, including glioblastoma (a type of brain cancer), endometrial (uterine) tumors, and prostate cancer, as well as in Cowden disease cells. PTEN stands for "phosphatase and tensin homolog". As well as having phosphatase activity, PTEN is similar to tensin, a protein that interacts with actin filaments at sites of intense signaling activity on the inner surface of cells known as focal adhesions.
PTEN taken from tumors often has a disabling mutation in the phosphatase domain, showing that it is important for normal PTEN function. But what might PTEN's substrate be? Although PTEN can act on both proteins and lipids in vitro, its favorite physiological substrate appears to be phosphatidylinositol 3,4,5-trisphosphate (PIP3), a lipid signaling molecule.
PIP3 is generated by the enzyme phosphoinositide kinase (PI3-kinase), which itself is activated by stimulatory signals emanating from the cell surface, often from focal adhesions. PIP3 activates yet another kinase called PKB/Akt, a proto-oncogene product. If PTEN fails to deactivate PIP3 because of a mutation in its phosphatase domain, downstream signals are not switched off, and therefore PKB/Akt remains in the "on" state. In this case, PKB/Akt can continue to stimulate downstream proteins such as transcription factors and glucose transporters, which could lead to enhanced cell growth.
PTEN is not exclusively a human protein. In the worm Caenorhabditis elegans, a PTEN homolog seems to help control lifespan and dauer formation (a hibernation state). Use of such animal models will help further investigate PTEN and could give clues to outstanding questions, such as how PTEN itself is regulated.
Search PubMed for the function of tensin
Created: November 10, 1999
Click on the link below to start an html tutorial.
What does tensin do?
Use BLAST to search for proteins similar to PTEN
Created: November 10, 1999
Click on the link below to start an html tutorial.
Search for proteins similar to PTEN
- PTEN and the tumor suppressor balancing act - Coffee BreakPTEN and the tumor suppressor balancing act - Coffee Break
Your browsing activity is empty.
Activity recording is turned off.
See more...