NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Dean L, McEntyre J, editors. Coffee Break: Tutorials for NCBI Tools [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 1999-.
Obesity contributes to poor public health in many Western populations, underlying illnesses that range from cardiovascular disease to hypertension and stroke. Over the past years, research into animal models of obesity has teased apart some of the endocrinological pathways that mammals have evolved to regulate the body's fat content in times of feast and famine. Now, new insight into the subtle workings of these pathways comes from a paper in the September issue of Nature Genetics, which reports the phenotypic effects of inactivating the gene that encodes the mouse melanocortin receptor 3 (Mc3r).
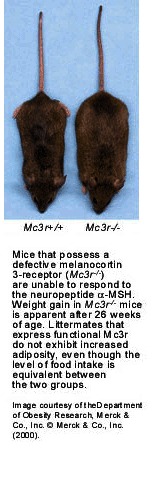
Mc3r-/- mice appear to grow normally up to 26 weeks of age, but although at this age they are not overtly obese, their fat mass is almost double that of wild-type and heterozygote littermates. This is because their increased fat mass is initially obscured by a compensatory decrease in lean muscle mass. It is only after 26 weeks that their increased weight gain becomes more obvious (see picture). Unexpectedly, this increased adiposity is not caused by increased food intake. Instead, Mc3r-/- mice gain more fat per calorie of food consumed, apparently at the expense of their lean body mass. This so-called increased feed efficiency means that the mutant mice store more fat despite eating less than normal mice do, and they become obese if fed a high-fat diet. The mechanism behind these responses is unclear because the Mc3r-/- mice have normal metabolic rates, body temperatures, and thyroid function. However, they are less active than wild-type mice, which might contribute to their tendency to obesity, and they also show a transient reduction in neuropeptide Y levels. Because this hypothalamic neuropeptide has been implicated in feeding-control mechanisms, Chen et al. suggest that its reduction in Mc3r-/- mice may contribute to their reduced food intake.
So how do Mc3r-/- mice differ from Mc4r-/- mice, and what does this tell us about the different functions of the two receptors in the control of food intake and energy expenditure? Mc4r-/- mice eat more than normal mice and are obese. They also have altered metabolic rates and a normal lean body mass. When Chen et al. treated Mc3r-/- mice with a non-selective melanocortin agonist, it reduced food consumption in both mutant and normal mice to a similar degree, indicating that α-Msh probably inhibits food intake by acting through Mc4r. Further evidence supporting distinct functions for these two receptors came when Chen and colleagues crossed the two knock-out mice to produce double homozygote mutants, which were more obese than mice lacking just Mc4r. In an accompanying News and Views article, David Cummings and Michael Schwartz speculate that this phenotype occurs because the double mutants eat excessively, because of the loss of Mc4r signalling, and store consumed calories more efficiently, because of the absence of both receptors.
These new insights into the functions of Mc3r could contribute to the development of new diagnostic and therapeutic approaches to treating obesity disorders in humans, and further research should clarify whether drugs that act through Mc3r and Mc4r could be used therapeutically to reduce food intake and its storage as fat.
Story contributed by Jane Alfred, Nature Reviews Genetics
Search for proteins similar to leptin
Created: November 6, 2000
Click on the link below to start an html tutorial.
Search for proteins with tertiary structure similar to leptin
- OMIMRelated OMIM records
- The mouse that eats less but gains weight - Coffee BreakThe mouse that eats less but gains weight - Coffee Break
Your browsing activity is empty.
Activity recording is turned off.
See more...
