NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Dean L, McEntyre J, editors. Coffee Break: Tutorials for NCBI Tools [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 1999-.
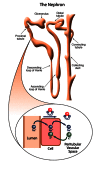
The human genome lays down the blueprint for our physiology and thus provides a framework by which to study genetic-based diseases. Researchers have focused recently on a region that may be responsible for a debilitating kidney disease — autosomal dominant renal Fanconi syndrome (RFS). In RFS, the proximal tubules of the kidney are functionally impaired. This causes many essential compounds that would normally be returned to the bloodstream to instead be excreted into the urine and removed from the body.
Genetic as well as environmental factors can lead to the development of RFS. An autosomal dominant form of RFS has been observed in several families; one of these was used as the basis for an attempt to find a genetic locus to the disease. The inheritable form of the syndrome is of particular interest for researchers because it can potentially provide insight into the workings of the proximal tubules.
Correlating a disease with a genetic mutation is not an easy task. To successfully map a disease gene on the human genome, it is necessary to have a series of genomic landmarks. This has been one of the major accomplishments of the Human Genome Project; over 10,000 polymorphic markers have been identified and contextually placed onto framework maps.
To find the region associated with RFS, DNA samples from the afflicted family were initially scanned using polymorphic markers that were distributed throughout the genome. Linkage analysis implicated marker D15S659, which is found on the long arm of chromosome 15. This initial hit gave researchers a rough area to further scrutinize by conducting a more detailed screen with 24 localized markers. From the secondary screen, two markers—D15S182 and D15S537 —were determined to exhibit the greatest correlation with RFS.
Genes in the 15q15.3 region are now being considered as candidates for association with RFS. By definitively associating autosomal dominant RFS with a gene, new insights into the pathology of the disease can be gained. One possible candidate gene, HSPC129, codes for a hypothetical protein with unknown function.
Further clues as to the possible function of an uncharacterized protein can be discerned by comparing it with other, characterized proteins (e.g., using NCBI's Related Sequences feature). In the case of HSPC129, one of the proteins it shares similarity with is the yeast protein Psr2P. This protein is involved in the indirect regulation of transmembrane sodium transportation. Active transport of ions such as sodium takes place in the proximal tubules of the kidneys and is a key component of healthy kidney function. Could HSPC129 also be involved in the regulation of ion transport in the kidneys similar to Psr2P regulation of sodium transportation in yeast cells? Although the presence of HSPC129 in the proximal tubules of the kidneys remains to be determined, studies on Psr2P in yeast may give insight into human HSPC129 function and possibly lead to a treatment for autosomal dominant RFS.
- (1) Original Paper
- (2) The first marker's location in the genome
- (3) Secondary screen implicates two markers
- (4) HSPC129 gene in Entrez Gene
- (5) Sequence of the hypothetical protein
- (6) Related sequences
- (7) Psr2P, a yeast gene
- (8) Function of Psr2P
- GeneLocus Links
- Finding Fanconi - Coffee BreakFinding Fanconi - Coffee Break
Your browsing activity is empty.
Activity recording is turned off.
See more...